News & Media-2014
Home / News & Media / 2014
2014
NEWS & MEDIA:


Media Contacts:
Mike Drummond
Masimo
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Announces Global Launch of Multigas Monitoring for the Root® Patient Monitoring and Connectivity Platform
ISA OR+ MOC-9 Module Supports Anesthesia Agent and Ventilation Management
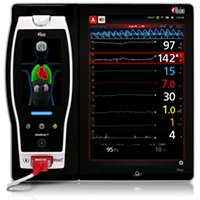
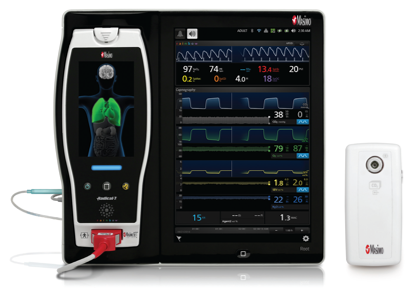 Masimo's ISA™ OR+ Multigas Monitoring for Root® measures anesthetic agents, O2, N2O, CO2, and respiration rate.
Masimo's ISA™ OR+ Multigas Monitoring for Root® measures anesthetic agents, O2, N2O, CO2, and respiration rate.
Irvine, Calif. – December 16, 2014 – Masimo (NASDAQ: MASI) today announced FDA 510(k) clearance and CE Mark of ISA™ OR+ multigas monitoring, a Masimo Open Connect (MOC-9) Module for the Root® patient monitoring and connectivity platform. During general anesthesia, the ISA OR+ monitors the inhaled and exhaled concentration of five anesthetic gas agents (Sevoflurane, Isoflurane, Halothane, Desflurane, Enflurane), carbon dioxide (CO2), nitrous oxide (N2O), and oxygen (O2), in addition to respiration rate. When technology modules are connected with Root, multiple additional parameters are available including Masimo SET® pulse oximetry, noninvasive and continuous hemoglobin (SpHb®), PVI®, SedLine® brain function monitoring, and O3TM Regional Oximetry.*
Earlier in 2014, Masimo announced the availability the ISA CO2 MOC-9 module, which provides end-tidal CO2 concentration and respiration rate.
Key features and benefits of ISA OR+ include:
- Requires only 50 ml sampling flow to support monitoring
- Time-saving in critical situations with virtually no warm-up time and full accuracy performance in less than 20 seconds
- Automatic anesthetic agent identification
- Supports monitoring patients with high respiration rates, up to 150 bpm
- Low-power consumption and automatic temperature and pressure compensation
- Provides minimal alveolar concentration (MAC), which is calculated from the measured anesthetic agents and N2O
- Appropriate for monitoring adult, pediatric, or infant patients in a range of clinical environments including the operating room and intensive care unit
- Compatible with Masimo's Nomoline Adapter and the Nomoline Airway Adapter Set to interface with endotracheal tubing.
*O3 Regional Oximetry is FDA 510(k) pending and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo ISA OR+ provides clinicians with accurate and continuous inhaled anesthetic agent monitoring for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Happy Holidays from Masimo!
Irvine, California — December 15, 2014 — In celebrating Masimo's 25th anniversary this year, the team showcased its commitment to innovation and patient care by launching a clinically significant product a month, including, Root, Radius-7, O3, ORI, and Eve. Also this year, breakthrough clinical studies showed that PVI can help with ERAS and reduce length of stay of patients in hospitals, and the largest-ever study of newborn screening for congenital heart disease showed that Masimo SET pulse oximetry significantly increased the rate of CHD detection and is reliable for detecting major congenital heart disease.
As we honor this very special time of the season, we continue our tradition to show you our gratitude by allowing you to give to the organizations that share our vision for a better world, in your name. Simply respond with an email to [email protected] specifying your charity choice from the list below and we will donate $10 in your name:
- Amnesty International
- CARE
- Clinton Foundation
- Doctors Without Borders
- Huntington's Disease Society of America
- Make-a-Wish Foundation
- March for Babies
- Masimo Foundation for Ethics, Innovation, and Competition in Healthcare
- Opportunity International
- PATH
- Patient Safety Movement Foundation
- SOS Children's Villages
- Last Mile Health
- UNICEF
- United Way
- World Vision
Once again, we are excited to be the presenting sponsor of the annual Patient Safety, Science & Technology Summit, Jan. 23-24, featuring former President Bill Clinton as the keynote. We are grateful for the opportunity to have transformed healthcare and we thank each of you for your support in helping us to improve the lives of clinicians and patients we diligently serve. Here's to a New Year full of boundless possibilities!
Masimo Guiding Principles
- Remain faithful to your promises and responsibilities.
- Thrive on fascination and accomplishment and not on greed and power.
- Make each day as fun as possible.
- Strive to make each year better than the year before, both personally and for the Team.
- Do what is best for patient care.
NOTE: Only e-mails sent to [email protected] from official Livewire members will be processed. Please also include any comments or suggestions you might have that will help us to better fulfill our mission and adhere to our guiding principles.


Masimo iSpO2™ Pulse Oximeter Featured in Fitness Book by Tour de France Champion Greg LeMond
Irvine, California, Dec. 4, 2014 – Masimo(NASDAQ: MASI) today announced that its award-winning iSpO2™ pulse oximeter is featured in the upcoming book "The Science of Fitness: Power, Performance, and Endurance," by three-time Tour de France champion Greg LeMond and co-author interventional radiologist Dr. Mark Hom, available at Amazon.com and the Elsevier Store.

"The Science of Fitness" (by Elsevier, the world's largest scientific book publisher) explores physical performance and its relationship with oxygen and mitochondria - the "power plants" of human cells that consume oxygen as they turn fuel (food, sugar, glycogen, and body fat) into cell energy. Arguably one of the greatest American athletes of all time, LeMond, along with Dr. Hom, tested the iSpO2 to help confirm their ideas about oxygen and to evaluate its feasibility on athletes.
"All athletes, from weekend warriors to Tour champions, get their energy from mitochondria and oxygen," said Dr. Hom. "We were able to test the iSpO2 device and found it to be very accurate, repeatable, and extremely easy to use. The iSpO2 is capable of recording and uploading graphical output of simultaneous pulse rate and oxygen saturation (SpO2) so we could analyze it later. It was small enough that we could test it on the road or when climbing stairs. Although a healthy person will not desaturate to the point of cyanosis (turning blue) even with rigorous training, we were able to see O2 saturation fluctuations in response to warm-up, intense activity, active recovery, hyperventilation, breath holding, and super-intense activity."
iSpO2, available for Android™ and Apple mobile devices, uses Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry to provide accurate, real-time oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI) readings - ideal for anyone who desires access to accurate fitness data through their mobile iSpO2. Extremely lightweight at just 232 grams or about 0.5 pounds, iSpO2 also displays the pleth waveform and Signal IQTM so users can visually assess their pulse rate and measurement quality to help optimize workouts and fitness levels. iSpO2 can also trend, store, and email up to 12 hours of measurement history in a global standard .CSV file format, allowing consumers to easily share data through their mobile device email application.

The iSpO2 consumer version is not intended for medical use. Please visit iSpO2.com for purchasing information.
Android is a trademark of Google Inc.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the iSpO2, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


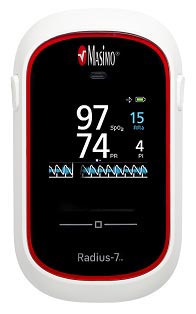
Masimo Announces FDA 510(k) Clearance of Radius-7™ – First rainbow® SET® Noninvasive Wearable, Wireless Monitor for Root®
Continuous, Untethered Monitoring of Oxygenation and Respiration Designed to Mobilize Patients

The wearable, wireless Masimo Radius-7™ offers continuous, noninvasive patient monitoring with comfort and freedom of movement.
Irvine, Calif. – December 1, 2014 – Masimo (NASDAQ: MASI) today announced FDA 510(k) clearance of Radius-7™ for the Root® patient monitoring and connectivity platform, the first and only wearable, wireless monitor with Masimo's breakthrough rainbow® SET® technology, offering patients continuous monitoring with freedom of movement.
With rainbow® SET® noninvasive measurements, Radius-7 with Root can alert clinicians – at the bedside or remotely, through the Masimo Patient SafetyNet™ remote monitoring and notification system – of critical changes in a patient's oxygen saturation and pulse rate – even during states of motion and low perfusion – as well as respiration through acoustic respiration rate (RRa®). Lightweight at only 0.34 lbs. (155g), the Radius-7 attaches to the patient's arm or can be placed alongside the patient in their bed, allowing untethered monitoring while they are in bed or out. With no need to disconnect and reconnect the cable to get out of bed, the Radius-7 reduces the need for nursing assistance. And the Radius-7's wireless communication functionality – either short-range via Bluetooth back to Root or with upgradeable Wi-Fi for long-range communication – ensures the patient can be continuously monitored and connected to caregivers wherever they are in the hospital.
Studies have shown that patient mobility is a key factor in more rapid patient recovery.1 Radius-7 allows clinicians to continuously monitor their patients when they are mobile.

Masimo Radius-7™ with one module always charging while the other is on a patient.
1 Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation Vol 91, Issue 4, PP 536–542, April 2010
Masimo Radius-7™ offers continuous monitoring and mobility.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our assumptions that Radius-7™ enables continuous monitoring with unprecedented patient ambulation and independence; reduces the need for nursing assistance; and allows for greater continuous monitoring for improved patient safety for all patients; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]


New Clinical Study Shows Accuracy of Masimo Noninvasive Spot-Check Hemoglobin (SpHb®) in Trauma Patients
Irvine, Calif. – November 5, 2014 – Masimo (NASDAQ: MASI) announced today that a new clinical study published in the Journal of the American College of Surgeons showed that noninvasive spot-check hemoglobin (SpHb) measurements had good correlation with invasive laboratory hemoglobin measurements in trauma patients with the potential to speed clinical decision-making to improve care, patient safety, and cost of care.1
Hemoglobin is one of the most frequently ordered laboratory tests in trauma settings.2 Although invasive measurements are considered the standard of care, limitations include collecting blood samples, transport of samples to the laboratory, analysis, and communication back to the physician who ordered the test – all of which are time consuming steps and can delay patient assessment.3,4 Spot-check SpHb can supplement laboratory hemoglobin measurements by providing noninvasive and quick assessment of a patient's hemoglobin, along with oxygen saturation and pulse rate.
At the University of Arizona's Department of Surgery, Division of Trauma, Critical Care and Emergency Surgery in Tucson, Ariz., Dr. Joseph and colleagues evaluated trauma patients who presented at a Level 1 Trauma Center. Noninvasive hemoglobin measurements using a Pronto-7 (version 2.1.9) were compared with invasive hemoglobin measurements from venous blood samples on a laboratory hematology analyzer (Siemens Medical Solutions Diagnostics, Zurich, Switzerland). Noninvasive spot-check hemoglobin (SpHb) measurements were attempted on a total of 525 patients, with a success rate of 86% (n=450). SpHb was measured three times in each patient using the Masimo Pronto-7. The first measurement was obtained on presentation to the trauma unit while the second and third measurements were obtained at five-minute intervals, for a total of 1,350 spot-check SpHb measurements.
Of the 75 patients in which SpHb could not be measured, 36 had either nail polish, soot or tar on their fingers, 21 had difficulty with sensor fit as researchers had only one size sensor for this study, 10 had radiological interference due to external factors such as X-ray and ultrasound devices, and the remaining eight patients were either too anxious or agitated to record SpHb.
The study population's invasive hemoglobin was 11.5 ± 4.36 g/dL (Range 6 g/dL-16 g/dL) and the average SpHb was 11.1 ± 3.60 g/dL (Range 6.4 g/dL-16.3 g/dL). The bias and standard deviation of SpHb compared to invasive hemoglobin was 0.3 ± 1.3 g/dL. Thirty-eight percent (n=173) of the patients had invasive hemoglobin ≤ 8 g/dL on presentation; 12% (n=54) of patients received a blood transfusion and 8% (n=36) underwent emergency surgical intervention for bleeding.
Researchers stated: "After dichotomization of our patients into two groups, patient with hemoglobin ≤ 8 g/dL and patients with hemoglobin >8 g/dL, spot-check hemoglobin measurements were found to have a sensitivity of 95.4% and an accuracy of 76%." Researchers also noted that "Noninvasive spot-check (hemoglobin) measurement has a strong correlation with the invasive hemoglobin measurements (ICC=0.70; CI: 0.57-0.80) and excellent correlation between the three consecutive noninvasive spot-check (hemoglobin) measurements (ICC=0.90; CI: 0.87-0.94)."
The researchers also stated: "We conclude that this novel technology allows for immediate and accurate hemoglobin measurements in trauma patients," and "We believe this device has the potential to improve clinical care, patient safety, and the cost of care."
Pronto-7 is a monitoring device and is not intended to be used as a standalone diagnostic tool. SpHb measurements are intended to supplement invasive hemoglobin measurements and are not intended to replace them.
1 Joseph B, Pandit V, Aziz H, Kulvatunyou N, Zangbar B, Tang A, Keeffe TO', Jehangir Q, Snyder K, Rhee P. "Transforming Hemoglobin Measurement in Trauma Patients: Non-Invasive Spot Check Hemoglobin," Journal of the American College of Surgeons (2014), doi: 10.1016/j.jamcollsurg.2014.09.022.
2 Gehring H, Hornberger C, Dibbelt L, et al. Accuracy of point of care testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol Scand. 2002; 46:980-86.
3 Mokken FC, van der Waart FJ, Henny CP, et al. Differences in peripheral arterial and venous hemorheologic parameters. Ann Hematol 1996; 73:135-137.
4 Yang ZW, Yang SH, Chen L, et al. Comparison of blood counts in venous, fingertip, and arterial blood and their measurement variation. Clin. Lab. Haem. 2001; 23:155-159.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to assumptions that Masimo SpHb can accurately track and trend Hb changes in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]


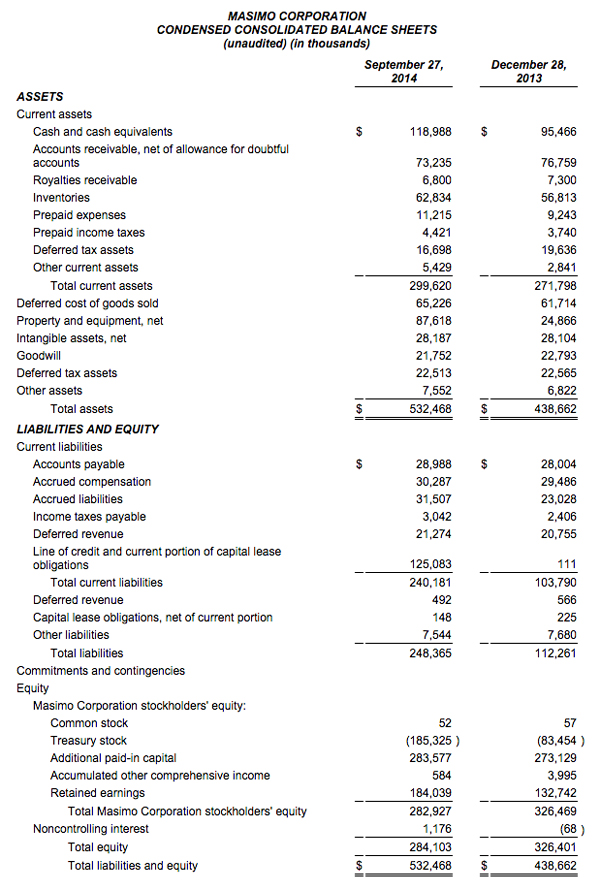
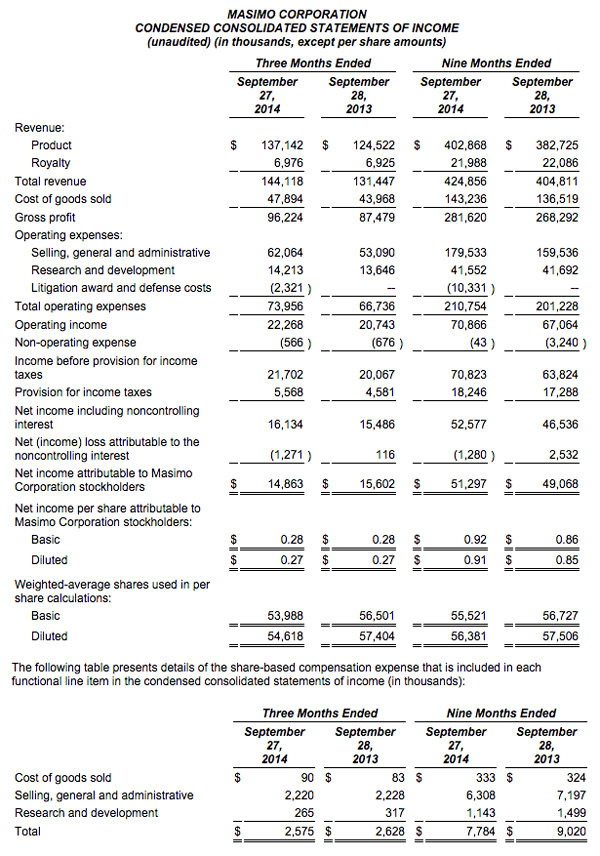
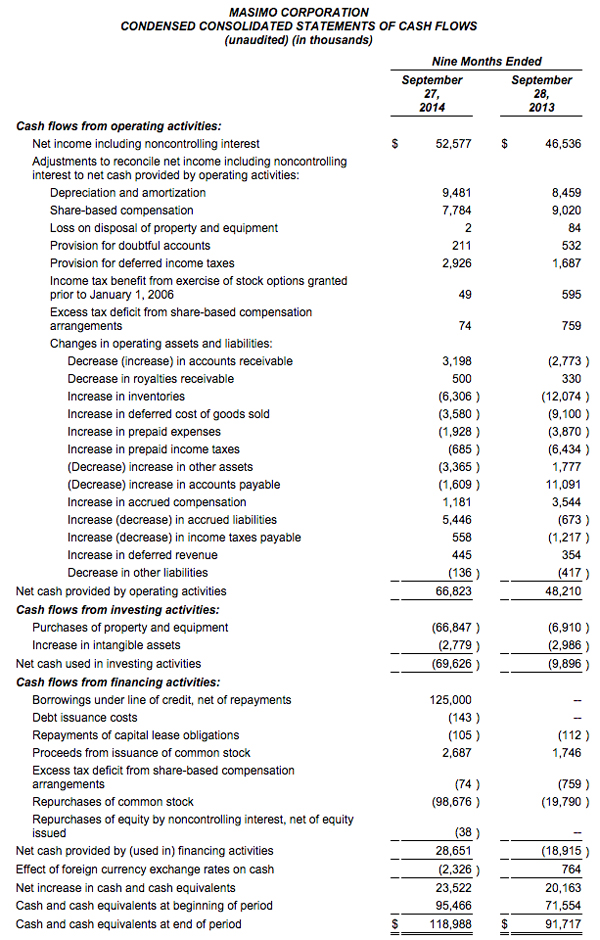
Masimo Reports Third Quarter 2014 Financial Results
IRVINE, CA -- (Marketwired) -- 10/29/14 -- Masimo (NASDAQ: MASI)
Q3 2014 Highlights (compared to Q3 2013):
- Product revenue rose 10% to $137.1 million
- Total revenue, including royalties, rose 10% to $144.1 million
- Masimo rainbow® revenue rose 10% to $13.2 million
- SET® and rainbow® SET® units shipments were 42,600
- Earnings per share was $0.27
Masimo (NASDAQ: MASI) today announced its financial results for the third quarter ended September 27, 2014.
The company's worldwide direct product revenue in the third quarter of 2014 rose by 9% compared to the same period in 2013 and represented 85% of product revenue. OEM sales, which accounted for 15% of product revenue, rose by 16% compared to the same period in 2013. Revenue from sales of Masimo rainbow products rose by 10% to $13.2 million in the third quarter of 2014, compared to $12.0 million in the prior year period.
Net income for the third quarter of 2014 was $14.9 million, or $0.27 per diluted share, compared to net income of $15.6 million, or $0.27 per diluted share, in the third quarter of 2013. During the third quarter of 2014, the company shipped approximately 42,600 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units. Masimo estimates its worldwide installed base as of September 27, 2014 to be 1,289,000 units, up 9% from 1,180,000 units as of September 28, 2013.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "We are happy with the acceleration in our year over year as well as our sequential quarterly SET® revenue growth. In fact, for the first time since 2010, our third quarter SET® and total product revenues exceeded the prior sequential second quarter levels. We are also encouraged by the sequential improvement in our product gross profit margins which are beginning to reflect the benefit of our value engineering program over the past two years."
As of September 27, 2014, Masimo's cash and cash equivalents were $119.0 million, compared to $95.5 million as of December 28, 2013. Additionally, during the first nine months of 2014, the company borrowed $125.0 million on its line of credit. During the third quarter, the company repurchased approximately 2.4 million shares of stock for $52.7 million, leading to repurchases of approximately 4.4 million shares of stock for $101.9 million through the third quarter of 2014, with approximately 0.6 million shares remaining in our original 6.0 million share repurchase program announced on February 14, 2013. In addition, the Board recently authorized the repurchase of up to an additional 3.0 million shares.
2014 Financial Guidance
Masimo is providing updated 2014 financial guidance. Masimo now expects fiscal 2014 total revenue to be approximately $585 million, including product revenue of approximately $556 million and royalty revenue of approximately $29 million. This represents a decline in the prior guidance for total 2014 revenues of approximately $588 million to $593 million, which included product revenues of $560 million to $565 million and royalty revenue of approximately $28 million. Despite the reduction in total product revenue and partially offsetting increase in royalty revenue guidance, Masimo expects fiscal 2014 GAAP earnings per diluted share to be approximately $1.28, within the prior range of $1.24 to $1.30. The guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be For further reading visit: from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 22235539. After the live webcast, the call will be available on Masimo's website through November 21, 2014. In addition, a telephonic replay of the call will be available through November 21, 2014. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 22235539.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005,Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2014 total, product and royalty revenues and GAAP earnings per share; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation



Investor Contact:
Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]



Arkansas Heart Hospital Upgrades to Masimo SET® Pulse Oximetry, Resulting in Significant Reduction in False Alarms and Improved Patient Care and Satisfaction
Ability of Acoustic Respiration Rate (RRa®) to Rapidly Detect Respiratory Changes May Offer Early Identification of Potential Adverse Events
Little Rock, Ark., & Irvine, Calif., October 23, 2014 – Masimo (NASDAQ: MASI) today announced that Arkansas Heart Hospital – a nationally recognized and award-winning hospital dedicated to the prevention, diagnosis and treatment of cardiovascular disease – has upgraded hospital-wide to Masimo SET® pulse oximetry, leading to a dramatic reduction in false alarms and a significant increase in patient satisfaction.
Arkansas Heart Hospital embraces the patient-safety suggestions of leading health organizations, including the Anesthesia Patient Safety Foundation, the Joint Commission, and the Institute for Safe Medication Practices (ISMP), which recommend continuous oxygenation and ventilation monitoring in patients receiving opioid-based pain medications.1-3 Opioid analgesics are associated with adverse effects and cause respiratory depression in 0.50%, or 25 of every 5,000 post-surgical patients.4-8
Arkansas Heart Hospital officials say that the previous pulse oximetry technology they used (Nellcor) created too many false alarms – one of the top technology hazards in hospitals today, according to the ECRI Institute. Responding to actionable alarms is critical to prevent patient injury or death, but the frequency of false alarms can increase workload and desensitize clinicians to all alarms, putting patients at risk.
"We began to see a trend with alarms being turned off because of false alarms," said Jason Henry, Director of Respiratory Therapy at Arkansas Heart Hospital. "We implemented a program to increase the percentage of time that alarms were turned on, and after switching to Masimo we were able to achieve 100%."
"Patient movement and patients with poor perfusion had been causing a lot of the false alarms," said Chris Dent, Vice President of Clinical Services at Arkansas Heart Hospital. "Masimo SET technology reduced that dramatically – its accuracy really helped us with our alarm management."
"We began to see a trend with alarms being turned off because of false alarms," said Jason Henry, Director of Respiratory Therapy at Arkansas Heart Hospital. "We implemented a program to increase the percentage of time that alarms were turned on, and after switching to Masimo we were able to achieve 100%."
Patients at Arkansas Heart Hospital noticed the silence, which helped the hospital improve its Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores – a national, standardized, publicly reported 27-item survey instrument and data collection methodology for measuring patients' perceptions of their hospital experience.
"Quietness at night in the facility has a great impact on patient satisfaction," Henry said. "Patients rate us on the quietness of the environment and alarm management is key to that. We're very dependent on the accuracy of alarms, and Masimo has given our staff confidence that when alarms sound, something is truly happening with the patient."
Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology has been proven by more than 100 independent and objective studies and used on more than 100 million patients a year in leading hospitals worldwide, including the top 10 on the U.S. News & World Report Best Hospitals Honor Roll (2014-2015). Multiple studies have shown that Masimo SET® significantly reduces false alarms (specificity), and accurately detects true alarms (sensitivity) compared to non-Masimo SET® pulse oximeters.9,10
In addition, in a study at Dartmouth-Hitchcock Medical Center in a 36-bed orthopedic unit covering 2,841 patients and 9,978 monitored days over 10 months, the implementation of Masimo SET® bedside pulse oximetry and Masimo Patient SafetyNet™ resulted in:
- Over every 1,000 discharges, patients who were not continuously monitored had 3.4 rescue activations, while patients monitored continuously with Masimo SET® had 1.2 rescue activations – a 65% reduction.
- Over every 1,000 patient days, patients who were not continuously monitored had 5.6 ICU transfers, while patients monitored continuously with Masimo SET® had 2.9 ICU transfers – a 48% reduction.
- Dartmouth-Hitchcock estimated cost savings of $58,459 savings per patient not transferred to the ICU, for an annualized savings over $1.5 million annually.11
"We are truly honored to work in partnership with Arkansas Heart Hospital, which has a proven commitment to patient care and safety," said Jon Coleman, Masimo President of Worldwide Sales, Professional Services and Medical Affairs. "We also are thrilled to see how use of our pulse oximetry technology has helped the hospital achieve its important alarm-management goals, while having the added benefit of increasing its patient-satisfaction scores."
1 Stoelting RK et al. APSF. 2011.
2 Joint Commission Sentinel Event Alert. Issue 49. August 8, 2012.
3 Institute for Safe Medication Practices
4 Vila H Jr, Smith RA, Augustyniak MJ: The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: Is patient safety compromised by treatment based solely on numerical pain ratings? Anesthesia and Analgesia, 2005;101:474-80.
5 Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Substance abuse treatment admissions involving abuse of pain relievers: 1998 and 2008, https://www.samhsa.gov/data/ (accessed October 28, 2011).
6 McPherson ML: Strategies for the management of opioid-induced adverse effects. Advanced Studies in Pharmacy, 2008;5(2):52-57.
7 Jarzyna D, et al: American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing, 2011;12(3): 118-145
8 Pasero C, M McCaffery: Pain assessment and pharmacologic management. Chapter 12 – Key Concepts in Analgesic Therapy, and Chapter 19 – Management of opioid-induced adverse effects. St. Louis, Mosby Elseveir, 2011
9 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers". J Clin Anesth. 2012 Aug;24(5):385-91.
10 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: here
11 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. https://www.apsf.org/newsletter/summer-spring-2012/
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET® provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Drew Jackson
Arkansas Hospital
Phone: (501) 219-7307
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Two New Clinical Studies Show Masimo Noninvasive Hemoglobin (SpHb®) Demonstrated Similar Precision as Hemoglobin from Point of Care Devices
Irvine, Calif. – October 16, 2014 – Masimo (NASDAQ: MASI) announced two new studies today that showed that noninvasive hemoglobin (SpHb®) demonstrated similar accuracy as hemoglobin measured by invasive hemoglobin analyzers when both SpHb and the invasive hemoglobin analyzer were compared to laboratory hematology analyzer.
The first study was presented at the American Society of Anesthesiologists (ASA) Annual Meeting in New Orleans, the largest gathering of anesthesiologists in the world, in patients undergoing potential high blood-loss surgery. Masimo SpHb showed similar absolute and trending accuracy as hemoglobin values determined by a laboratory blood gas analyzer, when both the SpHb and the hemoglobin from the blood gas analyzer were compared to hemoglobin from a laboratory hematology analyzer.
At the University of West Paulista (UNOESTE), Presidente Prudente, Brazil, Dr. Edmundo P. Souza Neto and colleagues compared absolute and trending accuracy of hemoglobin values from SpHb (Masimo Radical-7® Pulse CO-Oximeter and SpHb adhesive sensor, Revision K) and an invasive laboratory blood gas analyzer (Cobas B221, Roche Diagnostics, Indianapolis, USA) to hemoglobin values from a hematology analyzer (XE-2100, Sysmex, Kobe, Japan).1 While blood gas analyzers (also referred to as CO-Oximeters) and hematology analyzers are both laboratory devices that analyze blood samples to determine quantitative hemoglobin values, their methodologies are different and according to prior studies, their values are not interchangeable.2
In 33 patients, arterial blood gas samples were obtained 15 minutes after the incision, after each bleeding event (loss of at least 400 ml of blood in 40 minutes), and at the end of the surgery. A total of 69 arterial blood gas analyzer-determined hemoglobin values and 69 hematology analyzer-determined hemoglobin values were recorded. SpHb values were available continuously throughout the case and were recorded each time that arterial blood gas samples were obtained. Absolute accuracy was evaluated by comparing the SpHb and arterial blood gas hemoglobin values to the hematology analyzer hemoglobin values. The bias, or average difference, when compared to the hematology analyzer was -0.8 g/dL for SpHb and -1.4 g/dL for the invasive blood gas analyzer. The standard deviation, or maximum difference in approximately 68% of comparisons, was 1.3 g/dL for SpHb and 1.2 g/dL for the invasive blood gas analyzer. Outliers were defined as values >1 g/dL from hematology analyzer. There were fewer SpHb outliers (43%) than blood gas analyzer outliers (69%). Trend accuracy was evaluated by calculating the change in SpHb and change in hemoglobin from the blood gas analyzer and comparing the values to changes in hemoglobin from the hematology analyzer. The trend accuracy sensitivity of SpHb and the blood gas analyzer was determined by analyzing the percentage of time each method had the same directional trend as the hematology analyzer. There were no statistically significant differences between the two test methods for absolute accuracy (p=0.08) or for trending accuracy (p=0.6).
The researchers concluded: "Analysis of absolute accuracy showed a smaller bias but slightly larger standard deviation for SpHb than the blood gas analyzer when compared to the hematology analyzer reference. SpHb measurements had less outliers than blood gas analyzer and similar sensitivity to follow the correct directional sample to sample trend as determined by the reference. Further studies to increase sample size will be required to confirm these results and show potential differences between the test methods."
Separately, in a study published in the journal Anaesthesia and Intensive Care, Dr. R. Hiscock and colleagues at Mercy Hospital for Women in Heidelberg, Australia, studied the accuracy and repeatability of SpHb from a Masimo Pronto-7® spot-check device and a HemoCue Hb 201+ point of care invasive hemoglobin measurement device, compared to hemoglobin measured by a laboratory hematology analyzer (Sysmex XE-5000).3
For both devices, manufacturer instructions were followed to minimize recording variability and all readings were performed by a single operator, who collected an invasive venous sample and three SpHb measurements on 141 pregnant women aged 19 to 46 years. Venous samples were used to obtain three replicate hemoglobin measurements on the HemoCue Hb 201+ and a single hemoglobin measurement from a hematology analyzer. Compared to the hematology analyzer, researchers found a bias and standard deviation of 1.18 g/dL, ±1.19 g/dL for the Pronto-7 and 0.01 g/dL, ±1.34 g/dL for the HemoCue Hb201+. The Pronto-7 demonstrated higher repeatability than the Hemocue Hb 201+, as evidenced by a lower coefficient of variation % of (2.3% vs. 5.2%).
Researchers, who noted that neither device can replace laboratory-based hemoglobin analysis, concluded: "We found that the Pronto-7® device showed substantially better repeatability compared to the HemoCue® Hb 201+ device. Under stable conditions, we would be 95% certain that a repeat haemoglobin measurement using the Pronto-7® co-oximeter would lie within 0.82 g/dl of the previous reading, compared to 1.7 g/dl for the HemoCue® 201+."
Radical-7 and Pronto-7 are monitoring devices. They are not intended to be used as standalone diagnostic devices.
1 Neto E, Cursino de Moura Junior J, Laish J, Lalier Junior O, Mortatti P. Agreement of Noninvasive Hemoglobin Monitoring by Pulse CO-Oximetry (SpHb) with Invasive Laboratory Measurements. Proceedings of the American Society of Anesthesiologists, Oct.13, 2014, New Orleans, A3093, Room Hall B-1, Area C 2 Carabini LM, Navarre WJ, Ault ML, Bebawy JF, Gupta DK. "A Comparison of Hemoglobin Measured by Co-Oximetry and Central Laboratory During Major Spine Fusion Surgery." Anesth Analg. 2014 Sep 3. 3 Hiscock R, Simmons S, Carstensen B, Gurrin L. "Comparison of Massimo Pronto-7 and HemoCue Hb 201+ with laboratory haemoglobin estimation: a clinical study." Anaesthesia and Intensive Care, Vol. 42, No. 5, September 2014
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb can accurately track and trend Hb changes in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


New Clinical Study Presented at the American Society of Anesthesiologists Annual Meeting Shows Benefit of Oxygen Reserve Index™, ORI™
The Study Was Among 12 Selected from More Than 1,000 as One of ASA's Best Abstracts
Irvine, Calif. – October 15, 2014 – Masimo (NASDAQ: MASI) Masimo (NASDAQ: MASI) announced today that a new clinical study evaluating Masimo's latest noninvasive patient monitoring parameter, Oxygen Reserve Index ORI, showed that ORI can provide advanced warning of potential hypoxia and may help clinicians optimize oxygenation before and during prolonged intubation.1
The study was among 12 selected from more than 1,000 as one of the Best Abstracts at the American Society of Anesthesiologists (ASA) Annual Meeting in New Orleans, the largest gathering of anesthesiologists in the world.
At the University of Texas Southwestern and Children's Medical Center in Dallas, Dr. Peter Szmuk et al., used ORI to track oxygen available in the lungs during pre-oxygenation, safe apnea, and re-oxygenation. Investigators noted, "Since pre-oxygenation can cause oxygen saturation at 100% for variable durations, ORI would aid in the development of an advance indication of desaturation."1
Pulse oximetry (SpO2) provides noninvasive and continuous visibility to arterial blood oxygenation in hypoxia (less than normal oxygenation) and normoxia (normal oxygenation). During supplemental oxygen administration, clinicians often use the partial pressure of oxygen (PaO2), which is intermittent and delayed, to monitor levels of hyperoxia (higher than normal oxygenation). Between invasive sampling, changes in PaO2 cannot be assessed and therefore unexpected hypoxia or unintended hyperoxia can occur.
ORI, Masimo's 11th rainbow® parameter2, provides real-time visibility to oxygenation status in moderate hyperoxic range (PaO2 of approximately 100 to 200mmHg). ORI is intended to supplement, not replace, SpO2 monitoring and PaO2 measurements. As an "index" parameter with a unit-less scale between 0.00 and 1.00, ORI can be trended and has optional alarms to notify clinicians of changes in a patient's oxygen reserve.
With IRB and parental consent, researchers included for analysis 17 pediatric patients scheduled for surgery under general anesthesia with orotracheal intubation.
The mean time (±SD) from the start of the ORI alarm to SpO2 98% was 40±S52 seconds. During re-oxygenation, the time from SpO2 98% to stop of the ORI alarm was 65±S31 seconds.
"An advanced predictor of desaturation would be of great benefit to perioperative monitoring," researchers said. "The ORI alarm provides an increased warning time for avoiding potential hypoxia and could help in optimizing the oxygenation before and during prolonged intubation."
"Masimo congratulates Dr. Szmuk and his colleagues for the achievement and recognition they received at ASA this year," said Masimo founder and CEO Joe Kiani. "Their evaluation of ORI lays the groundwork for other clinicians to utilize this useful noninvasive parameter, designed to improve clinical decision-making and patient outcomes."
Radical-7® with ROOT has a CE Mark with the ORI parameter and is not FDA cleared and is not available for sale in the United States.
1. Szmuk P, Steiner J, Olomu P, Dela Curuz J, Sessler D. Oxygen Reserve Index - a New, Noninvasive Method of Oxygen Reserve Measurement" Proceedings of the American Society of Anesthesiologists, Oct.14, 2014, New Orleans, BOC12, Room 275-277 2. 11 parameters include: 1) oxygen saturation (SpO2); 2) Pulse rate; 3) Perfusion index (PI); 4) Pleth Variability Index (PVI); 5) Respiration Rate from the pleth (RRp); 6) Total hemoglobin (SpHb); 7) Oxygen Content (SpOC); 8) Carboxyhemoglobin (SpCO); 9) Methemoglobin (SpMet); 10) Fractional oxygen saturation (SpfO2); 11) Oxygen Reserve Index (ORI)
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo ORI offers noninvasive, continuous patient monitoring enabling full-time visibility to dissolved arterial oxygen status that may enable proactive interventions in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Retrospective Study of Enhanced Recovery After Surgery Program for Patients Undergoing Colorectal Surgery, which Included Fluid Therapy Tailored to Masimo's PVI®, Showed Reduced Length of Stay and Cost Savings
Findings Presented at American Society of Anesthesiologists Annual Meeting
Irvine, Calif. – October 14, 2014 – Masimo (NASDAQ: MASI) announced today the results of a retrospective study of an Enhanced Recovery After Surgery (ERAS) program in colorectal surgery patients, which included Masimo's PVI® monitoring. The ERAS program, which included fluid therapy tailored to PVI, resulted in reduced length of hospital stay, significantly reduced hospital costs, lower fluid administration, lower morphine administration, and earlier return of bowel function. The study was presented at the American Society of Anesthesiologists (ASA) Annual Meeting in New Orleans, the largest gathering of anesthesiologists in the world.
In the retrospective study of the ERAS program implemented at the University of Virginia, Dr. Robert H. Thiele and colleagues compared the results of 108 patients managed with the ERAS program to 98 consecutive patients before the ERAS program was implemented. The ERAS program included goal-directed therapy with PVI, ingestion of a carbohydrate drink two hours prior to surgery, pre-operative multimodal analgesic regimen, intraoperative low-dose spinal morphine, limiting intraoperative opiates, intraoperative infusions of ketamine and lidocaine (continued 48 hours post-operatively), early mobilization, and oral intake post-operatively.
Patients whose care was guided by the ERAS program with PVI had less fluid administered (973 ml vs 3,000 ml, p<0.001), lower morphine equivalents (0.1 vs. 20, p<0.001), earlier return of bowel function (p<0.03), lower pain score (2.33 vs. 4.85, p<0.001), fewer days of hospitalization (3 vs 5 days, P<0.001), and lower hospital costs ( $15,150 vs, $18,017 P<0.01).
The investigators concluded: "While this program includes several measures, most significantly patients received markedly less fluid and opiates in the operating room. Our program is novel in the use of PVI to guide therapy. This work highlights the importance of selection of anesthesia technique in determining outcomes for patients. Our ongoing work is in applying the lessons learned from this program to patients undergoing other types of surgery."
1 Colquhoun D, Turrentine F, Rea K, Friel C, Hedrick T, Thiele R. Implementing a Health System Wide Enhanced Recovery Program for Patients Undergoing Colorectal Surgery – The Anesthesiologists Perspective. Proceedings of the American Society of Anesthesiologists, Oct.12, 2014, New Orleans, A2010, Room 245
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET®outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our assumptions that PVI may help clinicians noninvasively and continuously assess fluid status of patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Announces CE Mark of Eve™ Newborn Screening Application for Radical-7, an Intuitive Software Feature to Help Clinicians More Effectively Screen for Critical Congenital Heart Disease
Neuchatel, Switzerland – October 10, 2014 – Masimo (NASDAQ: MASI), maker of breakthrough Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry, today announced the CE Mark of Eve™ Newborn Screening Software Application* for Radical-7® Pulse CO-Oximeters. Eve is designed to help clinicians more effectively and efficiently screen newborns for critical congenital heart disease (CCHD).

Eve™ Newborn Screening Application for Radical-7® Pulse CO-Oximeter
Newborn screening protocols may sometimes present challenges, among them: longer-than-necessary monitoring times, misapplication of sensors, calculation errors, and confusion interpreting results.
The Eve Newborn Screening Software Application in the Radical-7 Pulse CO-Oximeter automates the screening steps with animated instruction, including sensor application, measurement selection, and screening result determination.
Institutions can also choose to add the Perfusion Index (PI) measurement available in all Masimo SET® pulse oximeters to the screening criteria, which has been shown to identify CCHD or other illnesses not identified by physical exam or by SpO2 measurements alone.1
Use of the Eve Newborn Screening Software Application is intended to:
- Provide consistent application of the screening protocol to reduce method and operator-induced variability
- Improve efficiency by automating the data capture and comparison between readings.
CCHD is an umbrella term for a group of severe heart defects. All forms of CCHD affect the flow of blood into, out of, or through the heart, and if not detected and treated soon after birth can be deadly. CHD causes up to 3% of all infant deaths in the first year of life.2 According to the U.S. Department of Health and Human Services (HHS), congenital heart defects affect up to 9 of every 1,000 live births, one quarter of which could be detected and potentially treated by measuring blood-oxygen saturation with Measure-through Motion and Low Perfusion Pulse Oximetry – a quick, noninvasive, and inexpensive test that helps clinicians screen for potential CCHD that may signal the need for additional testing before a newborn leaves the hospital.
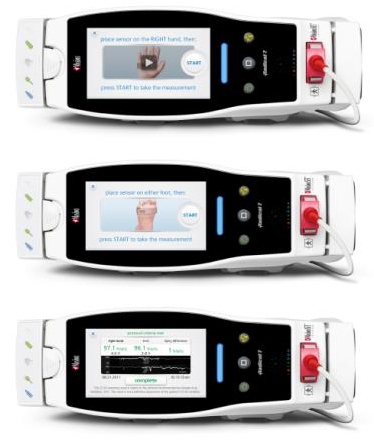
Eve™ Newborn Screening Application automates each of the screening steps with animated, instruction including sensor application, measurement selection, and screening result determination
In 2011, an expert workgroup formed under the auspices of the HHS Secretary's Advisory Committee on Heritable Disorders in Newborns and Children recommended newborn screening with Measure-through Motion and Low Perfusion Pulse Oximetry to increase the detection of CCHD.3 The CCHD workgroup cited the results of two large and independent prospective studies of 59,876 subjects that exclusively used Masimo SET® Measure-through Motion and Low Perfusion Pulse Oximetry4,5 to increase the identification of CCHD with minimal false positives.
In 2014, a third large, independent study of 122,738 newborns that also exclusively used Masimo SET® pulse oximetry showed similar, positive results as the first two large studies.6
Dr. Anne de-Wahl Granelli – lead author on the groundbreaking CCHD study, liaison to the U.S. Department of Health and Human Services Secretary's Advisory Committee on Heritable Diseases in Newborns and Children (SACHDNC) workgroup, and author of an award-winning doctoral thesis in this field – has stated: "While working at one of the two centers in Sweden that performed pediatric cardiac surgery, I found a significant difference, when compared simultaneously with blood gas, between Masimo SET and all other pulse oximetry technologies we used on cyanotic children in the PICU and pediatric cardiac ward. We were not aware of the significant difference between oximeters before this clinical study. And, as a result, we upgraded all our pulse oximeters to Masimo SET."
"Too many babies have been discharged from hospitals without proper pulse oximetry screening, only to die or require last-minute, emergency life-saving procedures," said Annamarie Saarinen, co-founder and CEO of the Newborn Foundation, and the mother of Eve, who was diagnosed at 48 hours old with CCHD. "Failing to follow and interpret a proper screening protocol, as well as being able to read measurements in newborns who are often in motion and have low perfusion are factors that can make CCHD screening with pulse oximetry a challenge. The data and study evidence show that having the right pulse oximetry technology for CCHD screening is critical – and it's very exciting to see the introduction of Eve, a new tool that offers nurses a clearer, safer, more efficient way to conduct newborns screening and protect newborn lives."
"When we started Masimo 25 years ago, the code name for our first technology, Measure-through Motion and Low Perfusion Pulse Oximetry, was Stork. We named it Stork because we had hoped that we could with this technology help clinicians and parents deliver babies safely to their home," said Joe Kiani, founder and CEO of Masimo. "Annamarie and her daughter Eve have inspired us to name the new automated CCHD detection application, Eve. Annamarie has worked tirelessly to make CCHD screening the standard of care worldwide. We salute her and Dr. Granelli, and hope that Eve will help these professionals accomplish their mission to help save the lives of babies and spare families from heartbreaking loss."
This application is not available in the United States.
* Referred to as "CCHD Mode" in Radical-7
1 de-Wahl Granelli A et al. Acta Paediatr. 2007 Oct;96(10):1455-9.
2 Secretary of Health & Human Services letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011. Available here.
3 Kemper, et al. Pediatrics. 2011
4 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338
5 Ewer AK et al. Lancet. 2011 Aug 27;378(9793):785-94
6 Zhao Q-m, Ma X-j, Ge X-l, Liu F, Yan W-l, Wu L, Ye M, Liang X-c, Zhang J, Gao Y, Jia B, Huang G-y, Neonatal Congenital Heart Disease screening group. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. The Lancet, Early Online Publication, 23 April 2014. doi:10.1016/S0140-6736(14)60198-7
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET® improves CCHD detection in newborns before hospital discharge, risks related to our assumptions of the repeatability of clinical results obtained, and risks related to our assumptions that Masimo SET® pulse oximetry technology improves patient outcomes, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Announces CE Mark of the 11th rainbow® Parameter - ORI™, Oxygen Reserve Index™
First Noninvasive & Continuous Parameter to Provide Insight into Oxygen Reserve in Patients Receiving Supplemental Oxygen
Masimo's ORI™ parameter, as seen on the Root® patient monitoring display platform, offers real-time visibility to oxygenation reserve in patients receiving supplemental oxygen, which may enable proactive interventions.
Neuchatel, Switzerland – October 7, 2014 – Masimo (NASDAQ: MASI) today announced CE Mark and limited market release of Oxygen Reserve Index (ORI™), the first noninvasive and continuous parameter of its kind to provide insight into a patient's oxygen reserve when they are receiving supplemental oxygen. With ORI, Masimo's rainbow SET® Pulse CO-Oximeters with the latest MX-5 circuit board can now measure an unprecedented 11 parameters1 through its noninvasive optical rainbow® sensor technology.
Pulse oximetry (SpO2) provides noninvasive and continuous visibility to arterial blood oxygenation in hypoxia (less than normal oxygenation) and normoxia (normal oxygenation). During supplemental oxygen administration, clinicians often use the partial pressure of oxygen (PaO2), which is intermittent and delayed, to monitor levels of hyperoxia (higher than normal oxygenation). Between invasive sampling, changes in PaO2 cannot be assessed and therefore unexpected hypoxia or unintended hyperoxia can occur.
ORI provides real-time visibility to oxygenation status in moderate hyperoxic range (PaO2 of approximately 100 to 200mmHg). ORI is intended to supplement, not replace, SpO2 monitoring and PaO2 measurements. As an "index" parameter with a unit-less scale between 0.00 and 1.00, ORI can be trended and has optional alarms to notify clinicians of changes in a patient's oxygen reserve.
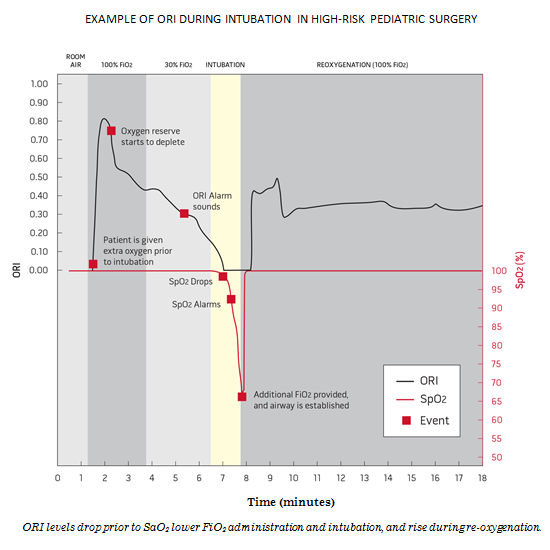
In patients receiving supplemental oxygen such as those in surgery, conscious sedation, or the intensive care unit, ORI may provide an advance warning of an impending hypoxic state, or an indication of an unintended hyperoxic state. In this way, ORI may enable proactive interventions to avoid hypoxia and unintended hyperoxia.
"ORI is another example of Masimo's commitment to take noninvasive patient monitoring to new sites and applications," said Masimo founder and CEO Joe Kiani. "We believe ORI will have significant applications during surgical procedures, intubation and procedural sedation, among others, and can help clinicians improve patient outcomes by keeping patients in the optimal oxygenation zone to help reduce risk for both hypoxia and hyperoxia."
ORI is available in Europe, but is not available in the United States.
1. 11 parameters include: 1) oxygen saturation (SpO2); 2) Pulse rate; 3) Perfusion index (PI); 4) Pleth Variability Index (PVI); 5) Respiration Rate from the pleth (RRp); 6) Total hemoglobin (SpHb); 7) Oxygen Content (SpOC); 8) Carboxyhemoglobin (SpCO); 9) Methemoglobin (SpMet); 10) Fractional oxygen saturation (SpfO2); 11) Oxygen Reserve Index (ORI)
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow®; Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo ORI offers noninvasive, continuous patient monitoring enabling full-time visibility to dissolved arterial oxygen status that may enable proactive interventions in all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation



Mercy Hospital of Buffalo Expands Use of Masimo Patient SafetyNet™ After Pilot Program Shows Reduction in Mortality
Ability of Acoustic Respiration Rate (RRa®) to Rapidly Detect Respiratory Changes May Offer Early Identification of Potential Adverse Events
Buffalo N.Y. & Irvine, Calif. – September 29, 2014 – Masimo (NASDAQ: MASI) and Mercy Hospital of Buffalo – one of the largest hospitals in Western New York – today announced the facility has expanded continuous patient monitoring using Masimo SET® pulse oximetry and the Masimo Patient SafetyNet™ remote monitoring and clinician notification system after seeing reduction in mortality on a pilot-program floor.
At Mercy, Masimo Radical-7 and Rad-87 devices continuously monitor oxygenation with Masimo SET® pulse oximetry and breathing with RRa. Patient Safety net enables the remote monitoring and wireless notification to clinicians of events detected by the bedside devices.
"After conducting a 30-month study of Patient SafetyNet on the hospital's 33-bed medical/surgical 6 McAuley West Unit, the hospital concluded that continuous patient monitoring can benefit all patients and potentially save lives, and so expanded the system throughout the hospital," said Jon Carlson, Mercy Hospital's Director of Respiratory Care.
"The outcome of the trial was a reduction in all-cause mortality on the pilot floor (excluding hospice and comfort care patients from both baseline and study data)," said Carlson. "The Patient SafetyNet monitoring system gives us the opportunity to further enhance patient safety, improve outcomes, and reduce costs by avoiding preventable patient transfers to the ICU."
Carlson noted that a key feature of the Patient SafetyNet system is Masimo SET with ability to measure through challenging patient conditions, including motion as well as low perfusion (or diminished blood flow).
Kathleen Guarino, Mercy Hospital's Vice President of Nursing and Chief Nursing Officer, said Patient SafetyNet is also helping the staff better manage patients' pain. "It provides a 'safety net' by constantly monitoring oxygen saturation and breathing, which is especially important for our patients using narcotic pain medication," she said.
"We are honored and humbled that our technology has helped Mercy Hospital achieve better patient outcomes," said Joe Kiani founder and CEO of Masimo. "We hope all hospitals one day will implement our technology for continuous monitoring of patients, which has been shown to save lives and reduce costs."
About Mercy Hospital of Buffalo
Mercy Hospital of Buffalo, part of Catholic Health, is one of the largest and busiest hospitals in Western New York. As home to the Catholic Health Heart Center, the hospital offers a full range of cardiac care services including heart surgery, cardiac catheterization and minimally-invasive cardiology procedures. A recognized leader in advanced stroke care, Mercy Hospital is the only local hospital to earn Comprehensive Stroke Center designation by The Joint Commission, the nation's leading accrediting body for hospitals and health providers. Mercy's Family Birthplace provides a full spectrum of maternity/child and neonatal intensive care services, as well as a specialized pediatric care unit. For more information, visit the Catholic Health website at https://www.chsbuffalo.org/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all patients at Mercy Hospital of Buffalo will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Melanie Griffis
Mercy Hospital of Buffalo
Phone: (716) 826-3747
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Announces MX-5 OEM Circuit Board, Offering Breakthrough rainbow® Pulse CO-Oximetry™ Measurements at 250mW
Low Power Consumption rainbow® Pulse CO-Oximetry Circuit Board Enables Design of Smaller, Lighter, More Portable Patient Monitoring Devices Equipped with the High Performance Capabilities of Noninvasive SpHb®, SpOC™, SpCO®, SpMet®, and RRa® Measurements
The Masimo MX-5 circuit board enables full functionality of Masimo rainbow® Pulse CO-Oximetry™ measurements at a fraction of the power consumption of prior rainbow® boards.
Irvine, California – September 25, 2014 – Masimo (NASDAQ: MASI) announced its new MX-5 OEM circuit board, a technology platform that utilizes approximately half the power of previously available rainbow® circuit boards to deliver breakthrough rainbow® Pulse CO-Oximetry noninvasive measurement performance. The MX-5 is also unique in that it can dynamically scale power consumption even further depending on the type of parameters being measured.
Original Equipment Manufacturers (OEM) use Masimo circuit boards to enable noninvasive Masimo SET® Measure-Through Motion and Low Perfusion™ pulse oximetry and rainbow® Pulse CO-Oximetry measurements in their own medical devices and patient monitors. Until now, enabling the powerful noninvasive measurement capabilities of rainbow® Pulse CO-Oximetry required 550 milliwatts of power on average. The reduced power consumption of the new MX-5 circuit board varies between 250 and 320 milliwatts of power on average, depending on the rainbow parameters being measured. This approximately 50% reduction in power consumption is expected to facilitate integration into next-generation patient monitoring solutions that require a more efficient power profile to enable smaller, lighter, and more mobile battery-operated devices.
In addition to the lower power demands compared to previous rainbow® technology boards, the MX-5 adds dynamic power utilization to scale the MX-5's power draw based upon the combination of parameters being monitored to permit even longer battery runtimes. For instance, when monitoring Masimo SET® SpO2 and pulse rate only, average power consumption of the MX-5 is reduced by more than 75% compared to the prior rainbow® technology board. Masimo MX-5 boards are the same size as their MX-3 predecessor, with dimensions of 2" x 2.5," and offer simple drop-in replacement.
Using just 50% of the previous power demand without compromising performance, Masimo MX-5 OEM boards offer full functionality of breakthrough rainbow® technology for noninvasive measurements of total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and acoustic respiration rate (RRa®), in addition to providing Measure-Through Motion and Low Perfusion oxygen saturation (SpO2), pulse rate, and perfusion index (PI) measurement capabilities of Masimo SET® pulse oximetry.
According to Masimo President of Worldwide OEM Business and Corporate Development, Rick Fishel, "The efficient MX-5 board represents Masimo's commitment to continuous technological improvement, which improves OEM ability to integrate rainbow's innovative noninvasive measurements into the design and development of smaller, more portable monitoring devices for a variety of existing and emerging health care applications. Circuitry innovations like the MX-5 allow our OEM partners to compete more aggressively in the marketplace by delivering the noninvasive rainbow Pulse CO-Oximetry capabilities that enable clinicians to make more timely decisions concerning patients' physiological status that may lead to better patient outcomes."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa®). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the high performance and low power consumption capabilities of the Masimo MX-5 circuit board; risks related to our belief that Masimo technologies improve patient outcomes and that the rainbow technology platform allows clinicians to noninvasively monitor multiple blood constituents, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo PVI® Helped Assess Fluid Responsiveness in Clinical Study of Adult, Noncardiac Patients
Neuchatel, Switzerland – September 19, 2014– Masimo (NASDAQ: MASI) announced today a recent study published in the Journal of Cardiothoracic and Vascular Anesthesia in which Masimo's noninvasive PVI® parameter helped clinicians assess fluid responsiveness in mechanically ventilated adult noncardiac surgery patients under general anesthesia.1
Clinicians commonly use fluid administration to improve hemodynamics in the perioperative period. Assessment of fluid responsiveness – the ability of the circulation system to increase cardiac output in response to volume expansion – is essential to guide fluid therapy and optimize preload.2 Too little fluid administration can result in low perfusion in peripheral tissue, but too much fluid administration can result in patients failing to respond to any amount of volume expansion,3,4, as well as fluid overload postoperatively.5,6 PVI, available with any Masimo SET® or rainbow® sensor, provides clinicians with a continuous, non-invasive measure for assessing fluid responsiveness. 7-11
In the study conducted at Box Hill Hospital and St. Vincent's Hospital in Victoria, Australia, Dr. Andy Sisnata Siswojo and colleagues assessed fluid responsiveness in 29 patients undergoing noncardiac surgeries. Investigators used a Masimo Radical-7 rainbow SET Pulse CO-Oximeter®, which automatically calculated and displayed PVI, and a minimally invasive esophageal Doppler monitor (CardioQ-ODM™, Deltex Medical).
Patients received intravenous volume expansion with 500 mL of colloid following induction of general anesthesia. Patients were classified into fluid responders and nonresponders based on a stroke volume index increase of ≥10%. There were 17 responders (59%) to the 500-mL volume expansion. The baseline PVI value was significantly different between responders and nonresponders (16.5 ± 6.4% v 10.3 ± 2.7%, P = 0.004). Researchers reported that the receiver operating characteristic analysis demonstrated significant predictive ability of an increase in stroke volume index for PVI with area under the curve of 0.84 (95% confidence interval = 0.69-0.99). The optimal cut-off value for baseline PVI was 10.5%, with a sensitivity of 88% and a specificity of 67%.
"In this study, patients presented for a wider range of low-risk noncardiac surgeries for which invasive monitoring would not be indicated," researchers said. "The advantage of the noninvasive nature of PVI in this setting is, therefore, very appealing."
The investigators concluded: "PVI is predictive of fluid responsiveness in adult patients under general anesthesia undergoing noncardiac surgery when they are mechanically ventilated, in sinus rhythm, and free of pulmonary disease and cardiac dysfunction. It may serve as a useful tool for guiding intraoperative fluid therapy in circumstances in which more invasive monitoring is not indicated."
1 Siswojo A, Wong D, Phan T, Kluger R. "Pleth Variability Index Predicts Fluid Responsiveness in Mechanically Ventilated Adults During General Anesthesia for Noncardiac Surgery." Journal of Cardiothoracic and Vascular Anesthesia. Published online ahead of print, Aug 2014 doi:10.1053/j.jvca.2014.04.010
2 Cannesson M: Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth 24:487-497,2010
3 Brandstrup B, TonnesenH, Beier-HolgersenR, etal: Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. AnnSurg 238:641-648,2003
4 Marik PE, Cavallazzi R, Vasu T, etal: Dynamic changes in arterial wave form derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit Care Med 37:2642-2647,2009
5 Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth. 2002;14(4):252–6.
6 Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid T. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641-8. doi:10.1097/01.sla.0000094387.50865.23.
7 Loupec T., Nandoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-Ill Patients." Crit Care Med 2011 Feb;39(2):294-9. For further reading visit: https://journals.lww.com/ccmjournal/Abstract/2011/02000/Pleth_variability_index_predicts_fluid.8.aspx.
8 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." Eur J Anaesthesiol. 2010 Jun;27(6):555-61. For further reading visit: https://www.ncbi.nlm.nih.gov/pubmed/20035228.
9 Feissel M., Kalakhy R., Badie J., Robles G., Faller J., Teboul JL. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009, Brussels, Belgium. For further reading visit: http://ccforum.biomedcentral.com/articles/10.1186/cc7369.
10 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." Br J Anaesth. 2008 Aug;101(2):200-6. For further reading visit: https://www.ncbi.nlm.nih.gov/pubmed/18522935.
11 Forget P, Lois F, De Kock M. "Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesth Analg. 2010 Oct;111(4):910-4. Published online http://journals.lww.com/anesthesia-analgesia/pages/default.aspx.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, and that PVI is a preferred noninvasive indicator of fluid responsiveness in children, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


![]()
Yale-New Haven Hospital Upgrades to Masimo SET® Pulse Oximetry for Improved Patient Outcomes
New Haven, Connecticut, & Irvine, California, September 4, 2014 – Masimo (NASDAQ: MASI) today announced that Yale-New Haven Hospital – a nonprofit, 1,541-bed tertiary medical center ranked among the best in the U.S. – has upgraded system-wide to Masimo SET® pulse oximetry, the standard-of-care at leading hospitals around the world.
Yale-New Haven Hospital joins a growing and distinguished global roster of health organizations using Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, clinically shown to virtually eliminate false alarms1 and help clinicians detect life-threatening events.2 Yale-New Haven Hospital's standardization to Masimo SET® pulse oximetry is in keeping with the healthcare organization's dedication to excellence in care and delivering the best possible results for its patients.
"Masimo's SET pulse oximetry technology reduces the measurement error found in other types of pulse oximeters, and in particular offers greater accuracy during states of patient low perfusion – an important consideration when patients are under anesthesia," said Dr. Douglas Vaughn, M.D., Senior Director of Anesthesia Operations and Medical Director of Perioperative Services at Yale-New Haven Hospital. "We've found SET pulse oximetry to be a valuable tool, both in the operating room and in the intensive care environment."
In addition, a recently published study in the Journal of Perinatology showed that after the neonatal intensive care unit (NICU) at Yale-New Haven Children's Hospital switched to Masimo SET® pulse oximetry, there was a 37% lower rate of severe retinopathy of prematurity (ROP), an eye disorder that can cause blindness in premature babies, and a 53% lower rate of ROP requiring surgery.3
Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology has been proven by more than 100 independent and objective studies and used on more than 100 million patients a year in leading hospitals worldwide, including the top 10 on the U.S. News & World Report Best Hospitals Honor Roll (2014-2015). Masimo SET® significantly reduces false alarms (specificity), accurately detects true alarms (sensitivity), and provides measurements when other pulse oximeters fail.1,2 Most importantly, Masimo SET® pulse oximetry has been clinically proven to improve patient outcomes and reduce cost of care by helping clinicians:
- Decrease retinopathy of prematurity (ROP) in neonates by avoiding giving too much oxygen based on inaccurate SpO2 measurements3,4
- Increase detection of critical congenital heart disease (CCHD) in newborns by accurately identifying low SpO2 measurements5,6
- Reduce ventilator weaning time by titrating FiO2 faster and reduce arterial blood gas measurements in the ICU through more reliable SpO2 measurements7 and
- Decrease rapid response activations and ICU transfers to save lives and costs in post-surgical patients on the medical-surgical floors through earlier identification of patients in distress through low SpO2 and abnormal pulse rate measurements.2,8
"We are honored to partner with Yale-New Haven Hospital, a highly esteemed healthcare system with a well-deserved reputation for quality care," said Masimo founder and CEO Joe Kiani. "We look forward to helping Yale-New Haven Hospital with its ongoing commitment to improved patient outcomes, while reducing cost of care."
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers" .J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: https://pubs.asahq.org/anesthesiology/article/132/3/602/108898/Impact-of-Pulse-Oximetry-Surveillance-on-Rescue
3 Bizzarro MJ, Li FY, Katz K, Shabanova V, Ehrenkranz RA, Bhandari V. Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit. Journal of Perinatology (2014) 34, 33-38; doi:10.1038/jp.2013.122; published online 26 September 2013
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
6 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
7 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
8 Anesthesia Patient Safety Foundation. APSF Newsletter. "No Patient Shall be Harmed by Opioid-Induced Respiratory Depression." 2011; 26(2):21-40. For further reading visit: https://www.apsf.org/article/no-patient-shall-be-harmed-by-opioid-induced-respiratory-depression/?swpmtx=71e5c1e438d2ec73a5ac910e8618bd22&swpmtxnonce=e1780a4799.
About Yale-New Haven Hospital
Yale-New Haven Hospital is a nationally recognized, 1,541-bed, not-for-profit hospital serving as the primary teaching hospital for the Yale School of Medicine. Yale-New Haven was founded as the fourth voluntary hospital in the U.S. in 1826. Today, the hospital's two New Haven-based inpatient campuses include Yale-New Haven Children's Hospital, Yale-New Haven Psychiatric Hospital and Smilow Cancer Hospital. YNHH has a combined medical staff of about 4,500 university and community physicians practicing in more than 100 specialties. YNHH's York Street campus and associated ambulatory sites are Magnet-designated by the American Nurses Credentialing Center.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET® provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mark D'Antonio
Yale-New Haven Hospital
Phone: (203) 688-2493
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Announces CE Mark and Limited Market Release of rainbow® DCI-mini™ – First Noninvasive Total Haemoglobin (SpHb®) Spot-Check Sensor for Infants and Small Children
Neuchatel, Switzerland – August 28, 2014 – Masimo (NASDAQ: MASI) today announced CE Mark, clearance in Japan, and limited market release of the rainbow® DCI-mini™, the first noninvasive haemoglobin (SpHb®) spot-check sensor for infants and small children (weight 3 to 30 kg). Paired with Masimo's handheld Pronto® device, the rainbow® DCI-mini sensors are designed to help clinicians quickly and easily spot-check haemoglobin levels, which may facilitate the identification of anaemia – a condition reflected by low haemoglobin when there are not enough red blood cells carrying oxygen to the tissues. In low-resource countries, an estimated 3.5 billion people are anaemic,1 making it one of the world's most common disorders.
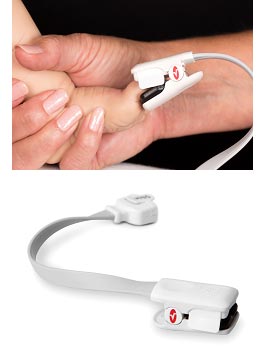
Masimo rainbow® DCI-mini™ noninvasive haemoglobin spot-check sensor for infants and small children.
Previously, SpHb spot-check sensors were available only for patients weighing 10 kg or more. Because iron deficient-anaemia during infancy and childhood has been shown to have long-lasting adverse effects on neurodevelopment,2 international health organizations recommend screening infants for anaemia between the ages of 9 to 12 months – an age that is often below 10 kg – with additional screening between the ages of 1 and 5 years for patients at risk.3,4
Masimo's rainbow® DCI-mini is a versatile sensor that is designed to facilitate proper placement and ease of use for clinicians and patients. The sensor uses a lightweight ribbon cable to connect to the Pronto device and a digit clip that is applied to a small child's finger or on an infant's big toe or thumb. The new rainbow® DCI-mini reusable spot-check sensor is ideal for clinics, public health programmes, and hospital emergency departments.
"The conventional process of drawing blood is a traumatic event, especially for younger patients, not to mention the cost," said Dr. Mohammed Bailony, an M.D. in paediatrics, who practices paediatric haematology-oncology at several hospitals in San Diego, including Scripps Mercy Hospital and Rady Children's Hospital. Dr. Bailony has screened patients for anaemia in developing nations and has used the rainbow® DCI-mini in clinical trials.
"SpHb assessment with the DCI-mini definitely will have a big role in developing nations, where laboratories are often not available," Dr. Bailony said. "In small cities, rural areas, and for mobile clinics, this will be a tremendous help in assessing patients. It's fast. It's friendly. And unlike needles, I haven't seen a kid who's scared of it."
"The DCI-mini allows clinicians and public health programmes around the world to expand haemoglobin assessment to vulnerable populations that need a noninvasive and convenient method," said Masimo founder and CEO Joe Kiani. "Every 90 seconds a woman dies from complications due to pregnancy and many of them due to anaemia. In addition children under 5 years old who suffer from anaemia do not develop fully, which has long term implications to the lives of those afflicted, as well as society in general. We hope that the new rainbow DCI-mini will help more infants and small children, along with their moms, around the globe to receive timely assessment and treatment, which will benefit their long-term health, as well as the health of our society."
The DCI-mini spot-check SpHb sensor is available in Europe, Japan and many other countries, but not available for sale in the United States, Canada, China, Singapore, Brazil and Mexico. For a more detailed list of country exceptions and available markets for sale, contact Masimo Customer Service.
1 United Nations Administrative Committee on Coordination Nutrition (ACC/SCN) 4TH Report on the world nutrition situation: Nutrition Throughout the Life Cycle. Sub-Committee on Geneva: ACC/SCN; 2000.
2 Baker R, Greer R, The Committee on Nutrition, "Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age)" PEDIATRICS Vol. 126 No. 5 November 1, 2010 pp. 1040 -1050
3 United Nations Children's fund, United Nations University and World Health Organization, "Iron Deficiency Anaemia, Assessment, Prevention, and Control – A Guide for Programme Managers" 2001: WHO/NHD/01.3. https://apps.who.int/iris/rest/bitstreams/1161313/retrieve
4 Kohli-Kumar M, "Screening for Anemia in Children: AAP Recommendations—A Critique" PEDIATRICS Vol. 108 No. 3 September 1, 2001 pp. e56
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including risks related to our assumptions regarding the repeatability of clinical results, risks related to our assumptions that Masimo SpHb accurately tracks and trends Hb changes in all patients, risks related to our belief that SpHb enables quick and easy noninvasive spot-checking of hemoglobin at the point-of-care for all patients, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


New Clinical Study Evaluates Masimo RAM™ Technology for Tracking Changes in Respiratory Rate in Anesthetized Patients
Ability of Acoustic Respiration Rate (RRa®) to Rapidly Detect Respiratory Changes May Offer Early Identification of Potential Adverse Events
Irvine, California – August 22, 2014 – Masimo (NASDAQ: MASI) announced today that a new study published in the journal Anesthesia & Analgesia demonstrates that Masimo's rainbow® Acoustic Monitoring (RAM™) technology for acoustic respiration rate (RRa®) rapidly detects changes in respiration rate during general anesthesia with a laryngeal mask airway and spontaneous ventilation, and is helpful in the early identification of patients at risk for adverse outcomes.1
Masimo RAM™ technology features an adhesive acoustic respiration rate (RRa) sensor applied to the patient's neck to continuously measure respiration rate.
In the study, Dr. Joshua Atkins, M.D., and colleagues from the Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania in Philadelphia, obtained complete data sets from 50 patients undergoing elective urological surgical procedures in the operating room. 1269 minutes of simultaneous data from the pneumotachograph signals and RRa were compared using a Bland-Altman analysis that showed the 95% limits of agreement were -2.1 to 2.2 breaths per minute with a mean error of 0.05 bpm (Bias/Precision of 0.1/1.1 bpm).
The authors report that while accurate monitoring of respiration rate may be useful for the early detection of patient deterioration, "Monitors such as capnometry and thoracic impedance pneumography have significant drawbacks." Known clinical drawbacks of capnometry include patient intolerance and high false alarm rates2; limitations of thoracic impedance pneumography include susceptibility to artifact, potential inaccuracy, and inability to detect apnea.3
"Under conditions of spontaneous ventilation during general anesthesia, RRa provides accurate estimates of respiratory rate changes over a wide range of respiratory rates," the researchers said, and noted that, "RRa is able to track changes in respiratory rate with minimal delay."
The authors concluded: "To the extent that immediate knowledge of changes in respiratory rate is beneficial in early identification of patients at risk for adverse outcomes, RRa may be a useful clinical monitoring indicator."
Masimo's RAM technology provides a method of continuous, noninvasive monitoring of respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck. Using acoustic signal processing that leverages Masimo's patented breakthrough Signal Extraction Technology (SET®), the respiratory signal is separated and processed to display continuous acoustic respiration rate (RRa) – enabling earlier detection of respiratory compromise and patient distress. Several studies have shown RAM is responsive to changes in respiration rate, has similar accuracy to capnography, is superior to capnography in detecting respiratory pause, and has greater patient tolerance than capnography in various patient populations.1,2,4,5
1 Atkins J, Mandel J. Performance of Masimo Rainbow Acoustic Monitoring for Tracking Changing Respiratory Rates Under Laryngeal Mask Airway General Anesthesia for Surgical Procedures in the Operating Room: A Prospective Observational Study. Anesthia-Analgesia Epub July 2014.
2 Ramsay M, Usman M, Lagow E, Mendoza M, Untalan E, De Vol E. "The Accuracy, Precision and Reliability of Measuring Ventilatory Rate and Detecting Ventilatory Pause by rainbow Acoustic Monitoring and Capnometry." Anesth Analg; April 30, 2013 ANE.0b013e318290c798. For further reading visit: http://www.anesthesia-analgesia.org/content/early/2013/04/30/ANE.0b013e318290c798.full.pdf+html.
3 Goudra BG, Penugonda LC, Speck RM, Sinha AC. Comparison of acoustic respiration rate, impedance pneumography and capnometry monitors for respiration rate accuracy and apnea detection during GI endoscopy anesthesia. Open J Anesthesiol 2013;3:74–9
4 Mimoz et al. Accuracy of Respiratory Rate Monitoring using a Non-Invasive Acoustic Method after General Anaesthesia. Br J Anaesth. 2012 May;108(5):872-5.
5 Patino et al. Accuracy of Acoustic Respiration Rate Monitoring in Pediatric Patients. Paediatr Anaesth. 2013 Sep 3.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results and performance of Masimo rainbow Acoustic Monitoring; our belief that the breakthrough acoustic respiration rate (RRa) capabilities of Masimo's proprietary rainbow acoustic monitoring technology will provide more accurate, precise, and reliable results over capnography in all patients and monitoring conditions – enabling clinicians to detect and treat respiratory compromise and patient distress earlier; and other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


New Study of Masimo Noninvasive and Continuous Hemoglobin (SpHb®) Monitoring Evaluates Trending and Accuracy in Pediatric Patients
Irvine, California – August 12, 2014 – Masimo (NASDAQ: MASI) announced today that in a new study published in the journal Anesthesia and Analgesia, Masimo's noninvasive and continuous hemoglobin (SpHb®) technology showed a positive correlation for trending when compared with invasive lab measurements in pediatric patients undergoing surgeries with potential for substantial blood loss.1
In the study, Dr. Mario Patino, M.D., and colleagues from the Department of Anesthesiology at Cincinnati Children's Hospital, assessed the trending and point accuracy of SpHb (Revision E Disposable L Sensor R1-20/25L and ReSposable Sensor R2-20/25)) in pediatric patients versus arterial blood samples analyzed on a hematology analyzer (Cell-Dyn Sapphire). They also evaluated the impact of SpHb Invivo Adjustment on accuracy comparisons.
Researchers collected 158 SpHb and laboratory hemoglobin (Hb) data pairs and 105 delta pairs (![]() SpHb and
SpHb and ![]() Hb) from 46 patients aged 2 months to 17 years with Hb ranging from 16.7 g/dL to 7.9 g/dL. Patient selection was limited to those undergoing surgical procedures associated with substantial blood loss, such as cardiac surgery, liver/small bowel transplantation, and multilevel spine surgeries.
Hb) from 46 patients aged 2 months to 17 years with Hb ranging from 16.7 g/dL to 7.9 g/dL. Patient selection was limited to those undergoing surgical procedures associated with substantial blood loss, such as cardiac surgery, liver/small bowel transplantation, and multilevel spine surgeries.
The primary goal of the study was to compare the magnitude and direction of change (trending) of SpHb versus that of the reference device. The authors found that the sensitivity of SpHb to correctly detect directional changes in Hb was 95% (95% CI = 0.83–0.99). The researchers calculated SpHb's correlation coefficient at r=0.76. Additionally, 95% of the individual data points were either in the upper right or in the lower left quadrant of the trending plots, showing changes in SpHb and Hb in the same direction.
Additionally, researchers found point accuracy bias and standard deviation of 0.4 +/- 1.3 g/dL and with Invivo Adjustment, 0.1 +/- 1.2 g/dL. The limits of agreement were -2.0 to 3.2 g/dL before Invivo Adjustment and -2.4 to 2.2 g/dL after Invivo Adjustment (P value = 0.04). Invivo Adjustment, a feature currently available in select European and other non-U.S. markets, allows clinicians to adjust the SpHb measurement to that of an initial blood sample analyzed with the clinician's invasive reference hemoglobin analyzer. This feature helps account for reference invasive instrument and individual patient variation.
The authors concluded that, "noninvasive, continuous Hb monitoring by Pulse CO-Oximetry (SpHb) displayed similar trending and absolute accuracy in pediatric patients undergoing a variety of surgical procedures associated with blood loss as has been found in adult surgical patients," adding that, "trending SpHb values has the potential to supplement detection of changes of Hb concentration by invasive blood draws."
SpHb is available with Masimo's rainbow® SET® monitoring platform, enabling the noninvasive assessment of multiple blood constituents and physiologic parameters that previously required invasive or complicated procedures, in addition to providing Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry. The rainbow® SET® platform – including RRa®, SpCO® and SpMet® – offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options.
"This study adds to the growing body of clinical evidence supporting the value of SpHb's use with pediatric patients," said Dr. Stephen Barker, M.D., Ph.D., Chairman of the Scientific Advisory Board of Masimo. "As with adult patients, SpHb can help clinicians noninvasively assess hemoglobin levels and trending, which has been shown to lead to better clinical decisions and improved patient outcomes."
1 Patino M, Schultz L, Hossain M, Moeller J, Mahmoud M, Gunter J, Kurth C. "Trending and Accuracy of Noninvasive Hemoglobin Monitoring in Pediatric Perioperative Patients" Anesthesia and Analgesia, doi: 10.1213/ANE.0000000000000369. For further reading visit: https://pubmed.ncbi.nlm.nih.gov/25036374/.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Reports Second Quarter 2014 Financial Results
Q2 2014 Highlights (compared to Q2 2013):
- Product revenue rose 3% to $133.5 million
- Total revenue, including royalties, rose 3% to $140.9 million
- Masimo rainbow® revenue was level at $11.6 million
- SET® and rainbow® SET® unit shipments were 43,400
- Earnings per share was $0.24, including a $0.03 charge for a charitable contribution that was announced in May 2014
Irvine, California, August 7, 2014 – Masimo (NASDAQ: MASI) today announced its financial results for the second quarter ended June 28, 2014.
Second quarter 2014 product revenues rose 3% to $133.5 million, compared to $129.6 million for the second quarter of fiscal year 2013, and total revenue, including royalties, rose 3% to $140.9 million, up from $137.4 million for the second quarter of fiscal year 2013.
The company's worldwide direct product revenue in the second quarter of 2014 rose by 5% compared to the same period in 2013 and represented 85% of product revenue. OEM sales, which accounted for 15% of product revenue, declined by 6% compared to the same period in 2013. Revenue from sales of Masimo rainbow products was level at $11.6 million in the second quarter of 2014, compared to $11.5 million in the year-ago period.
Net income for the second quarter of 2014 was $13.8 million, or $0.24 per diluted share, compared to net income of $17.0 million, or $0.30 per diluted share, in the second quarter of 2013. Net income for the second quarter of 2014 included an expense of $2.5 million, or $0.03 per share, for the previously announced contribution to the Masimo Foundation.
During the second quarter of 2014, the company shipped approximately 43,400 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units, an increase of 2% compared to the same prior-year period. Masimo estimates its worldwide installed base as of June 28, 2014 to be 1,260,000 units, up 10% from 1,148,000 units as of June 29, 2013.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Although our results for the second quarter were below our original Q2 expectations, it will not have a substantial impact on our full year financial guidance given that some rainbow® orders, including a large international rainbow® order, was shifted from Q2 into Q3. Also, I am happy to report that in June, Masimo received FDA clearance for the full featured Root™ patient monitor, which includes IRIS™ connectivity hub and MOC-9™ for additional measurements, and our Nomoline™ capnography and anesthetic agent ISA side-stream monitors, again demonstrating our ability to innovate new technologies that can help advance patient care."
As of June 28, 2014, Masimo's cash and cash equivalents were $97.1 million, compared to $95.5 million as of December 28, 2013. During first half of 2014, the company borrowed $75.0 million on its line of credit. This additional cash, along with net cash generated from operations, was used to repurchase approximately 2.0 million shares of stock for $49.2 million and to acquire the company's new worldwide headquarters building for approximately $56.0 million.
2014 Financial Guidance
Masimo is providing updated 2014 financial guidance. Masimo now expects fiscal 2014 total revenue to be approximately $588 million to $593 million, including product revenue of approximately $560 million to $565 million and royalty revenue of approximately $28 million. In addition, Masimo now expects fiscal 2014 GAAP earnings per diluted share to range between $1.24 and $1.30. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be For further reading visit: from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 77154954. After the live webcast, the call will be available on Masimo's website through September 5, 2014. In addition, a telephonic replay of the call will be available through August 22, 2014. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 77154954.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2014 total, product, rainbow and royalty revenues and GAAP earnings per share; our plan to announce multiple new products in 2014; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
-
Investor Contact:
Eli Kammerman
(949) 297-7077
[email protected] -
Media Contacts:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
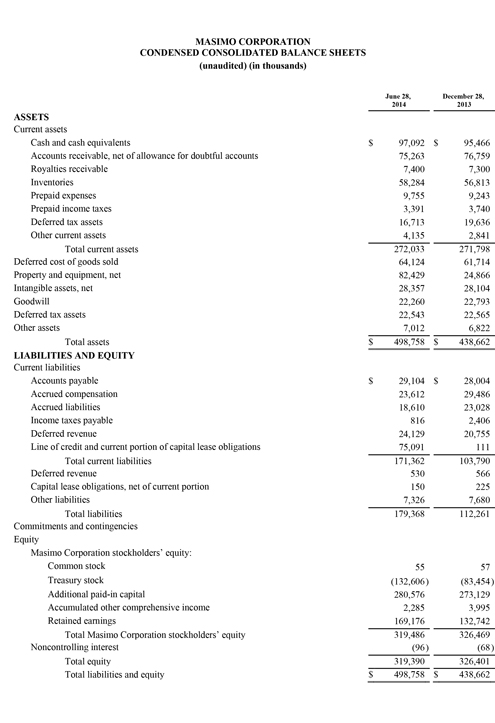
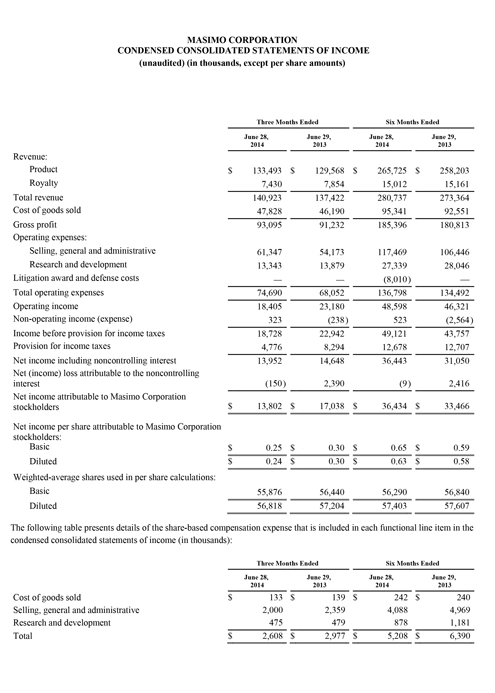
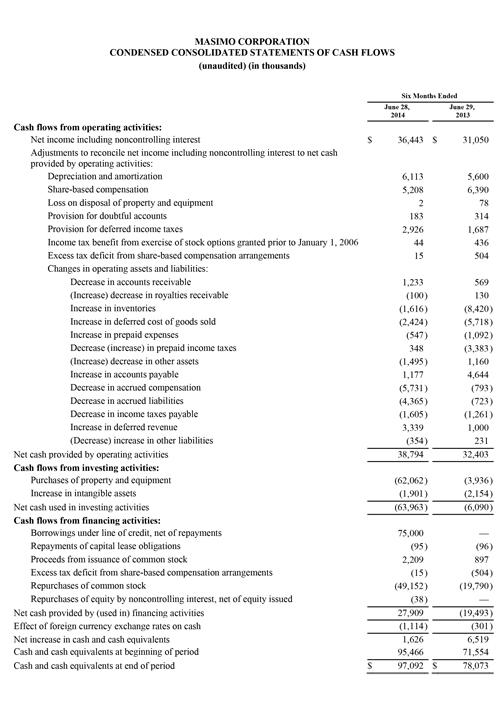





Masimo Announces CE Mark and Limited Market Release of Radius-7™ – First rainbow® SET® Noninvasive Wearable, Wireless Monitor for Root®
Continuous, Untethered Monitoring of Oxygenation, Circulation, Respiration, and Anaemia Designed to Mobilize Patients

The wearable, wireless Masimo Radius-7™ offers continuous, noninvasive patient monitoring with comfort and freedom of movement.
Neuchatel, Switzerland – July 29, 2014 – Masimo (NASDAQ: MASI) today announced CE Mark and limited market release of Radius-7™ for the Root® patient monitoring and connectivity platform, the first and only wearable, wireless monitor with Masimo's breakthrough rainbow® SET® technology, enabling early identification of clinical deterioration while offering patients continuous monitoring with freedom of movement.
See Radius-7 video.
With rainbow® SET® noninvasive measurements, Radius-7 with Root can alert clinicians – at the bedside or remotely, through the Masimo Patient SafetyNet™ remote monitoring and notification system – of critical changes in a patient's oxygen saturation, pulse rate, respiration rate, or haemoglobin that may indicate pulmonary, cardiac, or internal bleeding problems. Lightweight at only 155g (0.34 lbs), the Radius-7 attaches to the patient's arm or can be placed alongside the patient in their bed, allowing untethered monitoring while they are in bed or out. With no need to disconnect and reconnect the cable to get out of bed, the Radius-7 reduces the need for nursing assistance. And the Radius-7's wireless communication functionality – either short-range via Bluetooth back to Root or with upgradeable Wi-Fi for long-range communication* – ensures the patient can be continuously monitored and connected to caregivers wherever they are in the hospital.
Studies have shown that patient mobility is a key factor in more rapid patient recovery.1 Radius-7 allows clinicians to mobilize their patients.

Masimo Radius-7™ with one module always charging while the other is on a patient.
"With this new technology we have made another important step to improve patient safety," said Caroline Stade, chief nursing officer at University Children's Hospital Basel (UKBB) in Basel, Switzerland. "Children can be cared for and move about as they are able, while remaining under seamless, clinical surveillance. This makes parents, and of course, nurses and clinicians, feel better about the quality of care offered to our pediatric patients."
"In the past, wearable patient monitoring offered only limited measurements that were often plagued by false alarms. Radius-7 allows untethered clinically relevant patient monitoring," said Masimo founder and CEO Joe Kiani. "We expect Radius-7 and Root to change how hospitals think about monitoring, ushering in a new level of patient safety."
Radius-7 is not available for sale in the United States.
*The configuration for long-range communication is pending CE marking.
Masimo Radius-7™ offers continuous monitoring and mobility.

Masimo Radius-7™
offers Needham continuous
monitoring and mobility.
1 Needham D, Korupolu R, Zanni J, Pradhan P, Colantuoni E, Palmer J, Brower R, Fan E. "Early Physical Medicine and Rehabilitation for Patients With Acute Respiratory Failure: A Quality Improvement Project." Archives of Physical Medicine and Rehabilitation Vol 91, Issue 4, PP 536–542, April 2010
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our assumptions regarding timing of regulatory clearances and commercial availability in other countries; risks related to our assumptions that Radius-7™ enables early identification of clinical deterioration with unprecedented patient ambulation and independence; reduces the need for nursing assistance; and allows for greater continuous monitoring for improved patient safety for all patients; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Study Evaluates Trending Accuracy and Reliability of Masimo's Continuous and Noninvasive SpHb® Technology
Tokyo, Japan – July 10, 2014 – Masimo (NASDAQ: MASI) announced today that a new clinical study published in the Journal of Clinical Monitoring evaluated the relative trending accuracy of Masimo's noninvasive and continuous total hemoglobin (SpHb®) in patients with end-stage renal disease who underwent dialysis.1
Dr. Hiroshi Yamada, M.D., and colleagues at Fujisawa City Hospital in Fujisawa, Japan, assessed the relative trending accuracy of SpHb using a Masimo Radical-7 ® Pulse CO-Oximeter (ReSposable R2 25 Sensor, Rev. F) compared to in-line monitoring of hematocrit (Crit-Line) in end-stage renal disease patients undergoing dialysis. The researchers noted that while the Crit-Line device has proven to be clinically helpful, the high cost and low availability of the Crit-Line device has compelled clinicians to seek other options to assess changes in blood volume (ΔBV).
In the study, researchers determined the ΔBV estimated by each method by calculating the change between the initial reading of the modality and the current value at the time of recording. A total of 95 data points from 12 patients over 20 dialysis sessions were analyzed. The regression correlation of changes in SpHb vs. changes in hematocrit from Crit-Line was 0.83 (P≤ 0.001).
Researchers noted, "Pulse CO-Oximetry measures ΔBV from SpHb as reliably as Crit-Line," adding that, "SpHb by the pulse CO-Oximeter has good relative trending accuracy."
SpHb is available with Masimo's rainbow® SET® monitoring platform, enabling the noninvasive assessment of multiple blood constituents and physiologic parameters that previously required invasive or complicated procedures, in addition to providing Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry. The rainbow® SET® platform – including RRa®, SpCO® and SpMet® – offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options.
- Yamada H., Saeki M., Ito J., Kawada K., Higurashi A., Funakoshi H., Takeda K. "The relative trending accuracy of noninvasive continuous hemoglobin monitoring during hemodialysis in critically ill patients." J Clin Monit Comput May 3, 2014, DOI 10.1007/s10877-014-9574-6. For further reading visit: here
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Announces FDA Clearance of Root ™ Patient Monitoring Platform with Capnography, IRIS™ Standalone Device Connectivity, and MOC-9™ Third-Party Measurement Expansion
Irvine, California – June 30, 2014 – Masimo (NASDAQ: MASI) today announced FDA 510(k) clearance of the Root™ patient monitoring and connectivity platform that is destined to transform patient care throughout the hospital. High-impact innovations in Root that are now available in the U.S. include:

Root with ISA sidestream Capnography through the MOC-9 module
- Iris™ - Built-in connectivity gateway through Iris™ for standalone devices such as IV pumps, ventilators, hospital beds, and other patient monitors
- MOC-9™ - Flexible measurement expansion through Masimo Open Connect™ (MOC-9™) with MOC-9 modules from Masimo or third-party measurement by other companies to expand the platform's measurements and capabilities. New MOC-9 modules will require new 510(k) clearances
- Capnography - ISA™ CO2 sidestream module featuring fast warm-up time and the innovative and cost-effective Nomoline™ sampling line
- Wireless functionality – Capable of transmitting information through Bluetooth and Wi-Fi.
Masimo's breakthrough rainbow® and SET® measurements from the Radical-7® handheld monitor that docks into Root enable instant interpretation with a 10-inch-high visibility, intuitive navigation touchscreen display. In addition, the previously released SedLine brain function monitoring MOC-9 module for Root advances brain function monitoring to improve the care of patients under anesthesia or sedation.
Built-in Connectivity Gateway through Iris™
Despite medical technology advances, the lack of device communication and integration creates risks to patient safety in hospitals around the world. Without device interoperability, critical patient information can go unnoticed – leaving busy clinicians in the dark and vulnerable patients in danger. Existing approaches for device interoperability require separate hardware, software, and/or network infrastructure, which can clutter the patient room, increase complexity, burden IT management, and increase costs.
To address these challenges, each Root can be used as a connectivity gateway to connect multiple standalone devices – such as IV pumps, ventilators, hospital beds, and other patient monitors – when used as part of the optional Iris connectivity package in Masimo Patient SafetyNet™. Iris allows standalone device information to be remotely viewed with Patient SafetyNet, transmitted through notification systems or sent to electronic health record (EHR) systems to facilitate better patient care. Iris connectivity enables standalone devices to leverage existing network infrastructure and reduce costs while enhancing clinical workflows and decision support to improve patient safety, whether the clinician is at the bedside, down the hall, or on the next floor.
ISA Capnography MOC-9 Module
The ISA CO2 module for Root provides ETCO2 and respiratory rate measurements with crisp waveforms and fast warm-up time. In addition, customers can use the Nomoline™ "No Moisture" fluid protection sample line, which is specially designed for low-flow applications and excellent response time – making gas measurement possible even at high respiratory rates. Nomoline supports extended monitoring in low- and high- humidity environments to reduce disposable costs, and can be used for all types of patients from infants to adults.
MOC-9: Designed for Third-Party Development of Expanded Measurements
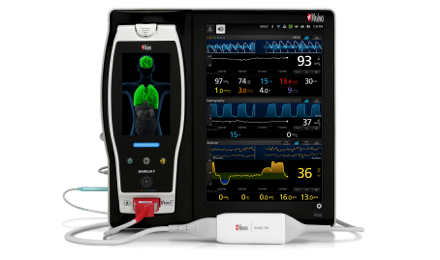
Root with Sedline brain functioning monitoring and ISA sidestream capnography
Root is also designed to allow other companies to expand the platform's measurements with their own measurements through MOC-9 by following Masimo's established development and validation process.
Market barriers and development costs often keep small, innovative companies from delivering their products to clinicians and patients who need them most. With Root's accessible patient monitoring platform, Masimo is offering an open invitation to other companies to develop and commercialize their innovations through Masimo's ever-expanding customer base.
Companies interested in developing a MOC-9 measurement can request more information online at: www.masimo.com/home/root/overview
"With the new FDA clearance for Root, Masimo is eager to help U.S. clinicians usher in a new era of patient care and improved patient safety with a platform that should measurably improve the performance and cost curve," said Joe Kiani, founder and CEO of Masimo. "Root can be a hub at the bedside, enable Masimo's breakthrough noninvasive measurements to be used by experts and novices with the trend and analog views, take advantage of a rich set of additional measurements, and allow other companies a robust platform on which to develop other innovative measurements via MOC-9."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our assumptions regarding timing of regulatory clearances and commercial availability in other countries; risks related to our assumptions that Root will provide a connectivity platform for any standalone device; and risks related to our assumption that Root will support/enable any third-party development of new measurements, technologies, product through MOC-9; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
Press Note: The included product photos illustrating various features and display screens on Root™ are available for media use and publication only.


Masimo Announces Support for the Every Newborn Action Plan to Reduce Global Newborn Mortality
Masimo's iSpO2 Rx Mobile Pulse Oximetry Technology Ideal for Early Detection of Health Conditions in Newborns in Low-Resource Settings
Irvine, California – June 27, 2014 – Masimo (NASDAQ: MASI) today announced its unconditional commitment to the Every Newborn Action Plan (ENAP), a global initiative to end preventable deaths of newborns. Coordinated by UNICEF and the World Health Organization, with the support of a broad group of partners, ENAP identifies actions for improving survival, health and development of newborns. Globally, about 3.3 million newborns die annually within the first month of life, with neonatal infection, sepsis, pneumonia and birth defects among the major killers, according to the World Health Organization.
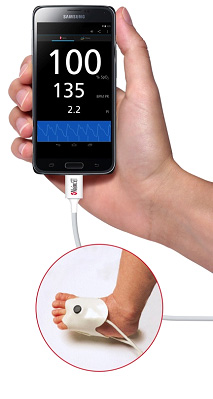
iSpO2 Rx Pulse Oximeter with M-LNCS connector
ENAP's mission is in lockstep with the commitments of Masimo, its longstanding partner the nonprofit Newborn Foundation, and the nonprofit Patient Safety Movement Foundation, to reducing preventable newborn deaths.
In late 2013, Masimo launched iSpO2 Rx, the first commercial, medical-grade Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximeter technology for mobile devices in conjunction with the Newborn Foundation's BORN Project – Birth Oximetry Routine for Newborns. iSpO2 Rx is ideally suited to low-resource areas. iSpO2 Rx for neonate and infant use is currently available in certain countries such as India, Cambodia, Kenya and Guatemala.*
Masimo and the Newborn Foundation spent nearly two years working with public health officials, delivery hospitals, and clinicians to create the first viable, measure-through motion and low perfusion, mobile-enabled pulse oximetry technology that can be adopted as part of routine neonatal screening for hypoxemia. In addition to Masimo's financial contributions to the BORN Project China, Masimo's engineering, design, and technical teams spent thousands of hours researching, designing and developing a mobile medical device that would serve the needs of health workers and babies in the lowest resource settings.
"We have found that this simple, noninvasive check of oxygen levels in newborns is among the most effective health measures that can be deployed to reduce newborn mortality," said Annamarie Saarinen, co-founder and CEO of the Newborn Foundation, who is scheduled to speak Monday at the ENAP Partner's Forum in Johannesburg, South Africa. "Through our partnership with Masimo, we are eager to help fulfill the goals of the ENAP initiative, saving and improving newborn lives."
Patient Safety Movement Foundation President Jim Bialick, also is scheduled to speak Monday at the ENAP Partner's Forum, and will address the need for easily accessible and highly reliable technologies to improve newborn healthcare worldwide.
The evidence for Masimo's pulse oximetry technology has never been more powerful. The largest newborn pulse oximetry study ever conducted was recently published in The Lancet.1 Led by a clinical team in Shanghai, more than 122,000 newborns were screened using Masimo SET® Measure-through Motion and Low Perfusion technology and found the use of Masimo SET® significantly increased the rate of congenital heart disease detection. The results corroborate those found in previous major CCHD studies in the United Kingdom and Sweden, including pioneering work by Dr. Anne de-Wahl Granelli, which showed that Masimo SET® pulse oximetry was effective in detecting newborns with CCHD with very high sensitivity and specificity.2,3 Now, the BORN Project China is providing the first large-scale newborn data on the efficacy of mobile pulse oximetry technology at county- and village-level birth facilities in Sichuan Province. China has among the highest newborn mortality as a percentage of under-5 deaths.
"Effective, affordable, and scalable pulse oximetry evaluation of newborns, along with reliable follow-up in low- and middle-income countries, will significantly reduce infection-related newborn death rates and improve outcomes for newborns afflicted with "hidden' congenital heart defects," said Masimo founder and CEO Joe Kiani. "Masimo SET iSpO2 Rx can arm front-line health care providers in the developing countries with the most effective pulse oximeter that is accurate in challenging conditions of patient movement and low perfusion to help save the lives of newborns."
iSpO2 Rx
iSpO2 Rx utilizes the same Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology proven by more than 100 independent and objective studies and used on more than 100 million patients a year in leading hospitals worldwide, including eight of the top 10 on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Masimo SET® significantly reduces false alarms (specificity), accurately detects true alarms (sensitivity), and provides measurements when other pulse oximeters fail.4,5 Most importantly, Masimo SET® pulse oximetry has been clinically shown to improve patient outcomes and reduce cost of care by helping clinicians:
- Decrease retinopathy of prematurity (ROP) in neonates by avoiding giving too much oxygen based on inaccurate SpO2 measurements6,7
- Increase detection of critical congenital heart disease (CCHD) in newborns by accurately identifying low SpO2 measurements2,3
- Reduce ventilator weaning time by titrating FiO2 faster and reduce arterial blood gas measurements in the ICU through more reliable SpO2 measurements8
- Decrease rapid response activations and ICU transfers to save lives and costs in post-surgical patients on the medical-surgical floors through earlier identification of patients in distress through low SpO2 and abnormal pulse rate measurements.5,9
* iSpO2 Rx for neonate and infant use is currently pending CE Mark and not available in the U.S. and Canada.
1 Zhao Q-m, Ma X-j, Ge X-l, Liu F, Yan W-l, Wu L, Ye M, Liang X-c, Zhang J, Gao Y, Jia B, Huang G-y, Neonatal Congenital Heart Disease screening group. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. The Lancet, Early Online Publication, 23 April 2014. doi:10.1016/S0140-6736(14)60198-7
2 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
3 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
4 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers" .J Clin Anesth. 2012 Aug;24(5):385-91.
5 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx.
6 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
7 Bizzarro MJ, Li FY, Katz K, Shabanova V, Ehrenkranz RA, Bhandari V. Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit. Journal of Perinatology (2014) 34, 33-38; doi:10.1038/jp.2013.122; published online 26 September 2013.
8 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
9 Anesthesia Patient Safety Foundation. APSF Newsletter. "No Patient Shall be Harmed by Opioid-Induced Respiratory Depression." 2011; 26(2):21-40. For further reading visit: https://www.apsf.org/article/no-patient-shall-be-harmed-by-opioid-induced-respiratory-depression/?swpmtx=71e5c1e438d2ec73a5ac910e8618bd22&swpmtxnonce=e1780a4799.
About Newborn Foundation
The Newborn Foundation is an international non-profit working specifically to leverage health IT and medical technologies to improve access and outcomes while reducing disparities for newborns. The organization has been integral in the policy development, adoption and implementation of technologies for early detection, intervention and care of the youngest patients, including universal newborn heart screening as a public health initiative.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the new iSpO2 Rx, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Jing Zhang
Newborn Foundation
Phone: (651) 414-1095
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Announces TFA-1™ Transflectance Adhesive Forehead Sensor
Irvine, California – June 25, 2014 – Masimo (NASDAQ: MASI) today announced CE Mark of the new TFA-1 transflectance forehead adhesive sensor, offering clinicians the power of Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry on an alternative monitoring site for rapid detection of oxygen saturation changes during low perfusion.
TFA-1 single-use sensors for adult and pediatric patients provide SpO2, pulse rate, perfusion index, and PVI® measurements.
TFA-1 sensors utilize Masimo SET®
Measure-through Motion and Low Perfusion™ pulse oximetry technology proven by more than 100 independent and objective studies and used on more than 100 million patients a year in leading hospitals worldwide, including eight of the top 10 on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Masimo SET® significantly reduces false alarms (specificity), accurately detects true alarms (sensitivity), and provides measurements when other pulse oximeters fail.2,3 Most importantly, the accuracy of Masimo SET® pulse oximetry in real-world conditions has been clinically proven to improve patient outcomes and reduce cost of care by helping clinicians:
Masimo TFA-1 transflectance single-use adhesive forehead sensors provide SpO2, pulse rate, perfusion index, and PVI measurements.
- Decrease retinopathy of prematurity (ROP) in neonates by avoiding giving too much oxygen based on inaccurate SpO2 measurements4,5
- Increase detection of critical congenital heart disease (CCHD) in newborns by accurately identifying low SpO2 measurements6,7
- Reduce ventilator weaning time by titrating FiO2 faster and reduce arterial blood gas measurements in the ICU through more reliable SpO2 measurements8
- Decrease rapid response activations and ICU transfers to save lives and costs in post-surgical patients on the medical-surgical floors through earlier identification of patients in distress through low SpO2 and abnormal pulse rate measurements.3,9
"The TFA-1 transflectance forehead adhesive sensor offers clinicians yet another way to leverage the breakthrough measurement capability in Masimo SET® pulse oximetry," said Joe Kiani, founder and CEO of Masimo. "By continuing to take Masimo's breakthrough technologies to new sites and applications, we are helping improve patient outcomes and safety while reducing cost of care."
TFA-1 transflectance forehead adhesive sensor is CE marked and available for purchase in CE countries.
1 Redford DT et al. Anesth Analg, 2004;98(2S):S-94.
2 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers" .J Clin Anesth. 2012 Aug;24(5):385-91.
3 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: https://pubs.asahq.org/anesthesiology/article/132/3/602/108898/Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
4 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
5 Bizzarro MJ, Li FY, Katz K, Shabanova V, Ehrenkranz RA, Bhandari V. Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit. Journal of Perinatology (2014) 34, 33-38; doi:10.1038/jp.2013.122; published online 26 September 2013
6 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
7 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
8 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
9 Anesthesia Patient Safety Foundation. APSF Newsletter. "No Patient Shall be Harmed by Opioid-Induced Respiratory Depression." 2011; 26(2):21-40. For further reading visit: https://www.apsf.org/article/no-patient-shall-be-harmed-by-opioid-induced-respiratory-depression/?swpmtx=71e5c1e438d2ec73a5ac910e8618bd22&swpmtxnonce=e1780a4799.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the new TFA-1 disposable transflectance forehead sensors, including our belief the sensors offer faster response to saturation changes compared to digit sensors during low perfusion, and our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Study of Masimo Noninvasive and Continuous SpHb® Monitoring Demonstrates Accuracy and Utility in Double-Jaw Surgery
Irvine, California – June 18, 2014 – Masimo (NASDAQ: MASI) announced today that in a new clinical study published in the Journal of Oral Maxillofacial Surgery, Masimo's noninvasive and continuous hemoglobin (SpHb®) technology provided clinically acceptable accuracy and helped determine when to perform invasive laboratory hemoglobin measurements in patients undergoing double-jaw surgery when massive hemorrhage is anticipated.1
In the study, Dr. Sung-Hoon Kim, M.D., et al., at the Asan Medical Center, College of Medicine, University of Ulsan in Seoul, Korea, compared SpHb measurements using SpHb Adhesive Sensors (Revision E) and the Masimo Radical-7® Pulse CO-Oximeter with laboratory hemoglobin measurements from a CO-Oximeter (Stat Profile pHOx Plus) in elective Le Fort I osteotomy and bilateral sagittal split ramus osteomy (BSSO) jaw surgery.
Of 51 patients, the average laboratory hemoglobin (tHb) and SpHb values were 12.6 ± 1.2 and 12.7 ± 1.6 g/dL before incision, respectively. These values decreased significantly over the course of the surgery to 10.0 ± 1.2 and 9.1 ± 1.3 g/dL at closure, respectively. The difference between the SpHb and tHb was less than 1 g/dL for 53 to 75% of measurements and less than 2 g/dL for 92 to 96% of measurements, respectively, at each time point. The difference between the SpHb and tHb data pairs was not affected by patients' mean arterial pressure, which was significantly decreased to a level of 50 to 60 mmHg during controlled hypotensive anesthesia.
The researchers stated that SpHb, "had a clinically acceptable bias compared with the conventionally measured tHb in the steady state, adding that, "Continuous monitoring of hemoglobin may help to determine the appropriate time to perform an invasive measurement of hemoglobin in patients undergoing double-jaw surgery when using controlled hypotensive anesthesia."
Background
Clinicians administer red blood cell transfusions based on a variety of factors including invasive hemoglobin measurements.2 However, laboratory measurements are intermittent and often delayed, leaving clinicians without any indication of hemoglobin status for extended periods of time.3
While SpHb monitoring is not intended to replace blood draws, it nonetheless identifies significant changes in trends and lack of significant changes in hemoglobin between invasive blood sampling and laboratory analysis. SpHb monitoring enables clinicians to determine the real-time, directional trend of hemoglobin – whether it is stable, rising, or falling. This can help clinicians avoid unnecessary transfusions when the SpHb trend is stable even though the clinician may otherwise perceive hemoglobin to be dropping, or when the SpHb trend is rising, but the clinician may otherwise believe that it's not rising fast enough. Inside and outside the operating room, a declining SpHb trend may also allow clinicians to identify internal bleeding and permit earlier interventions.4
SpHb is available with Masimo's rainbow® SET monitoring platform enabling the noninvasive assessment of multiple blood constituents and physiologic parameters that previously required invasive or complicated procedures, in addition to providing Masimo SET® Measure through Motion and Low Perfusion™ pulse oximetry. The rainbow® SET® platform – including RRa®, SpCO® and SpMet® – offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options.
1 Kim S-H, Choi J, Kim H, Choi S, Choi I. "Continuous Noninvasive Hemoglobin Measurement Is Useful in Patients Undergoing Double-Jaw Surgery." Journal of Oral Maxillofacial Surgery. Published online March, 31 2014. doi:10.1016/j.joms.2014.03.011. For further reading visit: https://www.joms.org/article/S0278-2391(14)00324-3/abstract
2 Zwart A, van Assendelft OW, Bull BS, et al: Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition). J Clin Pathol 49:271, 1996
3 Vos JJ, Kalmar AF, Struys MM, et al: Accuracy of non-invasive measurement of haemoglobin concentration by pulse COoximetry during steady-state and dynamic conditions in liver surgery. Br J Anaesth 109:522, 2012
4 Awada W.F.N., Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Study Shows Masimo Noninvasive SpCO® Helps Clinicians Increase Detection of Carbon Monoxide Poisoning in Emergency Department Patients
69% of Confirmed CO Poisoning Cases Undiagnosed without SpCO Assessment
Irvine, California – June 11, 2014 –Masimo (NASDAQ: MASI) today announced that a new study published in the International Journal of Clinical Practice demonstrated that Masimo's noninvasive SpCO® helped clinicians improve detection of carbon monoxide (CO) poisoning in patients presenting to a large emergency department.1 In the study, the reported prevalence of CO poisoning was nearly twice as high as reported in a previous study in the Journal of Emergency Medicine.2
CO poisoning is the leading cause of death from accidental poisoning, according to the Centers for Disease Control and Prevention. Lack of timely diagnosis can delay treatment or, worse, patients discharged with an incorrect diagnosis can be re-exposed to CO. CO poisoning accounts for an estimated 50,000 ED visits in the U.S. annually.3 Headaches are the most common symptom of CO poisoning – others include dizziness, nausea/vomiting, confusion, fatigue, chest pain, shortness of breath, and loss of consciousness. CO poisoning often is misdiagnosed and attributed to other illnesses such as the flu. Failure to diagnose CO poisoning can have disastrous consequences for patients and potentially other family members of affected households.4
In the 12-month study conducted July 2008 through June 2009 in the emergency department at Vienna General Hospital, in Vienna, Austria, Dr. Wolfgang Schreiber and colleagues prospectively assessed the prevalence of occult, or unsuspected, CO poisoning in 32,396 patients presenting to the ED. During the study period, noninvasive carboxyhemoglobin (SpCO) values using the Masimo Radical-7® Pulse CO-Oximeter were included as part of the standard vital-signs assessment. SpCO values exceeding 6% for non-smokers and 12% for smokers were validated by venous blood gas analysis with a laboratory CO-Oximeter (Radiometer ABL700).
The average noninvasive SpCO was 3.0% higher with smokers than non-smokers (4.0% vs. 2.0%, p<0.01). Among all patients, invasive COHb was measured in 2,377 individuals, similarly with smokers higher than non-smokers (2.9% vs. 0.7%, p<0.01). A total of 32 (1%) of the 32,396 patients presenting to the emergency department were diagnosed with CO poisoning –higher than the previously reported rates from both retrospective2,5-7, and prospective studies8,9. Of the 32 patients, 22 cases or 69% were occult and only identified due to SpCO assessment, which caused an invasive COHb test to occur and the CO poisoning diagnosis to be made.
"Our data on 32,396 prospectively screened ED patients, who received measurement of vital signs on triage, based on clearly stated definitions, and validated by blood gas analysis, suggest a prevalence of CO poisoning nearly twice as high as previous highest estimates," researchers stated. "This underlines the importance of this intoxication and appeals physicians for more alertness on the topic, especially as patients were unaware of CO poisoning in 22 out of 32 patients."
The researchers added: "The main consequence of testing for occult CO poisoning in an ED can be the prevention of potentially deadly re-exposure for patients, exposure for their adjacencies, as well as prevention of long term exposure that may lead to coronary artery disease, delayed neuropsychiatric syndrome, premature death," and "Screening of all patients using noninvasive technique might facilitate diagnosis, but an in-depth exposure history remains crucial."
SpCO is part of the Masimo rainbow® SET platform, which enables the assessment of multiple blood constituents and physiologic parameters that previously could only be measured invasively or with complicated procedures, in addition to providing Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry including SpO2, pulse rate, perfusion index, and PVI®. Multiple noninvasive and continuous measurements – including SpHb®, RRa® SpCO, and SpMet® – offer an advancement in patient safety by helping clinicians better assess patients.
"SpCO has been shown to be effective for screening large populations for CO poisoning, even when it is not suspected based on clinical presentation," said Dr. Steve Barker, M.D., Ph.D., Chairman of the Scientific Advisory Board of Masimo. "This large, prospective study further demonstrates early assessment with SpCO can help clinicians identify patients with unsuspected CO poisoning who are at serious risk for injury or death."
1 Roth D, Schreiber W, Herkner H, Havel C. "Prevalence of carbon monoxide poisoning in patients presenting to a large emergency department." Int J Clin Pract, March 2014; doi: 10.1111/ijcp.12432
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo SpCO will provide an accurate and effective noninvasive method of screening for CO poisoning, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website atwww.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation.


Two Randomized Trials Presented at the Euroanaesthesia 2014 Congress Show Similar Fluid Administration and Risk Profile with PVI as with Invasive or Complicated Procedures
Two Additional Studies Presented on Noninvasive SpHb in Postpartum Hemorrhage Detection and Oral Surgery
Irvine, California – June 6, 2014 – Masimo (NASDAQ: MASI) announced today that four new clinical studies evaluating Masimo noninvasive patient monitoring technologies were presented at the European Society of Anaesthesiology's (ESA) Euroanaesthesia 2014 Congress in Stockholm, Sweden.
PVI Studies
At University Hospital Linoping, in Linkoping, Sweden, researchers evaluated whether fluid volume optimization using PVI would lead to similar fluid management and patient risk compared to Stroke Volume Optimization using Esophageal Doppler (ED) – an established method to optimize preload during abdominal surgery. Researchers noted the ED technique "is costly and sensitive to interference, requires training and is not possible in prone position or at limited access to the head." The investigators reported that there were no differences in colloid administration, total volume of fluids given during surgery, or lactate levels at the end of surgery. Higher lactate levels are strongly associated with greater patient risk. The investigators concluded: "Fluid optimization during open abdominal surgery guided by PVI seems to result in equal amounts of fluid administered compared to guidance using ED technique."1
At CHU Brugmann (Brugmann University Hospital) in Bruxelles, Belgium, researchers compared conventional pulse pressure variation induced by mechanical ventilation (PPV) with noninvasive PVI to predict intraoperative fluid management in patients undergoing elective abdominal (laparoscopic) surgery. Seventy-two patients were randomized according to the monitoring used to guide intraoperative fluid therapy (PPV group: N=36, PVI group: N=36). Basal balanced crystalloid infusion rate was set at 2 ml/kg/h and boluses of 250 ml of 3% modified fluid gelatin were administered if the PPV was >13% or the PVI >15% in the respective groups for more than five minutes. Researchers concluded: "The type of monitoring does not influence significantly the volume of fluid administered in the intraoperative period."2
SpHb Studies
At Citta di Roma Hospital in Rome, Italy, researchers compared noninvasive SpHb with values from an invasive central laboratory device (Horiba Pentra DX 120) in laboring mothers to evaluate whether SpHb (rainbow® ReSposable R2-25 Revision K sensor; Radical-7 Pulse CO-Oximeter®) could detect changes in hemoglobin to enable earlier detection of postpartum hemorrhage. The investigators reported: "SpHb demonstrated bias and precision of 0.10 ± 0.71 g/dL compared to the central laboratory device with limits of agreement of 1.51 and -1.31 g/dL. More importantly, SpHb was able to trend changes detected by laboratory readings." They concluded that in this study: "SpHb was able to detect changes in hemoglobin concentration during and after delivery and therefore may provide a means for the early detection of bleeding and postpartum hemorrhage."3
At Tokyo Dental College Ichikawa General Hospital in Chiba-ken, Japan, researchers evaluated the accuracy of SpHb compared with laboratory CO-Oximetry measurements of total hemoglobin (tHb) during prolonged oral surgery. The investigators reported that 73 tHb values were compared to SpHb. Bias and precision were 0.86 g/dL and 1.17 g/dL, respectively. Bland-Altman analysis showed limits of agreement of -1.43 to 3.15 g/dl. They concluded: "The accuracy of SpHb monitoring during prolonged surgery was clinically acceptable, as shown by the low bias, precision and moderate limits of agreement when compared to laboratory values, although percent error exceeded normal range slightly."4
The Masimo rainbow® SET platform enables the assessment of multiple blood constituents and physiologic parameters that previously could only be measured invasively or with complicated procedures, in addition to providing Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry including SpO2, pulse rate, perfusion index, and PVI. Multiple noninvasive and continuous measurements – including SpHb, RRa®, SpCO® and SpMet® – offer an advancement in patient safety by helping clinicians better assess patients.
1 Bahlmann H., Hahn R., Nilsson L. "Pleth variability index as a tool for volume optimization during open abdominal surgery" Proceedings of the European Society of Anaesthesiology's (ESA) Euroanaesthesia 2014 Congress, June 2, 2014. Stockholm. 3AP5-5
2 Delaporte A., Ghoundiwal D., Bidgoli J., Foulon P., Vanderlinden P. "Goal directed fluid management" based on pleth variability index or pulse pressure variations during abdominal surgery" Proceedings of the European Society of Anaesthesiology's (ESA) Euroanaesthesia 2014 Congress, June 2, 2014. Stockholm. 4AP3-9
3 Tola G, Capogna G. "Noninvasive and continuous trending of hemoglobin during labor and in the post-partum period" Proceedings of the European Society of Anaesthesiology's (ESA) Euroanaesthesia 2014 Congress, June 2, 2014. Stockholm. 11AP3-1
4 Sazuka S., Koshika K., Watanabe Y., Ouchi T., Serita R., Koitabashi T. "Accuracy of continuous and noninvasive hemoglobin monitoring during prolonged surgery" Proceedings of the European Society of Anaesthesiology's (ESA) Euroanaesthesia 2014 Congress, June 2, 2014. Stockholm. 3AP4-3
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb®) and PVI® contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: immediate and continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


Masimo Patient SafetyNet™ and SpHb® Noninvasive Hemoglobin Monitoring Enable Clinicians to Meet New Guidance from Centers for Medicare & Medicaid Services
Masimo Technologies Proven to Help Improve Protection of Patients Receiving IV Opioids and Help Clinicians Better Manage Blood Transfusions
Irvine, California – May 21, 2014 –The Centers for Medicare & Medicaid Services (CMS) issued updated guidance for hospital medication administration – effective immediately – to reduce preventable deaths or serious adverse events related to intravenous (IV) opioid medications and blood transfusions.1 The CMS guidance "strongly encouraged" hospitals to review best practices from safety organizations, including continuous monitoring of oxygenation and ventilation for patients receiving IV opioids.
CMS's clarifications are provided for various provisions of 42 CFR 482.23(c), concerning medication administration, and 42 CFR 482.51(b)(4), concerning post-operative patient care.
An estimated one-third of all hospital adverse events are related to Adverse Drug Events (ADEs), affect approximately two million hospital stays annually, and prolong hospital length of stay by approximately 1.7 to 4.6 days.2 Each year, serious adverse events, including fatalities, associated with the use of IV opioid medications occur in hospitals, CMS noted in its memorandum. It also noted hospital patients on IV opioids may be placed in units where they typically are not monitored as frequently as in post-anesthesia recovery or intensive care units, increasing the risk that patients may develop respiratory distress that will not be immediately recognized and treated.
Masimo Patient SafetyNet™ has been clinically shown to reduce preventable and costly rescue events, transfers to intensive care units, and deaths related to opioid-induced respiratory depression.3 Patient SafetyNet combines the performance of Masimo SET® pulse oximetry – the enabler of reliable oxygenation and pulse rate monitoring in the general ward – with ventilation monitoring and wireless clinician notification that can help ensure patients' safety by noninvasively and continuously measuring and tracking their underlying physiological conditions and changes that signal declining health status in real-time. When changes occur in the measured values, which may indicate deterioration in the patient's condition, the system automatically sends wireless alerts directly to clinicians – prompting a potentially lifesaving response to the patient's bedside.
For example, since implementing Patient SafetyNet in 2007, Dartmouth-Hitchcock Medical Center in Lebanon, N.H., has had zero patients die or suffer permanent brain injury due to opioid-induced respiratory depression.
After expanding post-surgical monitoring to the general and thoraco-vascular post-surgical units, Dartmouth-Hitchcock reported:
- 57% overall reduction in rescue events over all units (4.4 to 1.9 per 1,000 patient days per month)
- 168 ICU days saved in the thoraco-vascular unit in the first 12 months after implementation
- 21% decrease in average length of stay of a patient with transfer to the ICU
- $1.48 million in annual opportunity cost savings due to decreased ICU transfer rate
- $58,459 saved per patient who was not transferred to the ICU
CMS referenced the Patient Safety Movement Foundation's Actionable Patient Safety Solutions (APSS): The "Patient Safety Movement Foundation (PSMF) recommends all patients receiving IV opioids have continuous measure-through motion and low perfusion pulse oximetry, and that patients on supplemental oxygen also have continuous respiration rate monitoring. It also calls for the monitoring system to be linked with a notification system to clinical staff who can respond immediately. It calls for an escalation protocol so that if a staff person does not acknowledge the alert in 60 seconds a second person will be notified."
CMS also noted safety measures from the Institute for Safe Medication Practices (ISMP) and the Anesthesia Patient Safety Foundation (APSF). ISMP makes available a list of high alert medications, which it defines as those medications that bear a heightened risk of causing significant patient harm when they are used in error. The current list may be found at:
http://www.ismp.org/Tools/highAlertMedicationLists.asp
Meanwhile, the APSF calls for every patient receiving postoperative opioid analgesics to be managed based on the following clinical considerations:
- Individualize the dose and infusion rate of opioid while considering the unique aspects of each patient's history and physical status.
- Make continuous monitoring of oxygenation (pulse oximetry) the routine rather than the exception.
- Assess the need for supplemental oxygen, especially if pulse oximetry or intermittent nurse assessment are the only methods of identifying progressive hypoventilation.
- When supplemental oxygen is indicated, monitoring of ventilation may warrant the use of technology designed to assess breathing or estimate arterial carbon dioxide concentrations. Continuous monitoring is most important for the highest risk patients, but depending on clinical judgment, should be applied to other patients.
The APSF video on opioid-induced ventilatory impairment can be seen at
https://www.apsf.org/videos/monitoring-for-opioid-induced-ventilatory-impairment-oivi-video/
While opioid use is safe for most patients, opioid analgesics are associated with adverse effects4,5,6 and cause respiratory depression in 0.5% of post-surgical patients, who often receive them for pain management.7,8,9 Opioid analgesics rank among the drugs most frequently associated with adverse drug events, according to The Joint Commission.10 Of opioid-related adverse drug events – including deaths – that occurred in hospitals and were reported to The Joint Commission's Sentinel Event database (2004-2011), 47% were wrong dosing medication errors, 29% were related to improper monitoring of the patient, and 11% were related to other factors including excessive dosing, medication interactions, and adverse drug reactions.
Regarding blood transfusions, CMS stated: "Whenever IV medications and blood transfusions are administered, the patient may become at risk for fluid and electrolyte imbalance. Hospital policies and procedures must address monitoring and treatment for fluid and electrolyte imbalances that may occur with blood transfusions and IV medications," and noted, "Blood transfusions can be life-saving. However, like IV medications, blood transfusions are not without risk of harm to patients. Transfusion reactions and/or errors can be fatal."
Masimo SpHb® noninvasive and continuous total hemoglobin can help hospitals adhere to CMS guidance. SpHb allows clinicians to noninvasively and continuously monitor hemoglobin blood levels, and provides real-time directional trends, such as indicating stable hemoglobin when it may be perceived to be dropping, and rising hemoglobin when it may be perceived to not be rising fast enough. SpHb monitoring has been shown to help clinicians reduce the number of risky and costly blood transfusions in surgical patients, and speed up blood transfusion for those who need it.11
"Masimo's noninvasive and continuous monitoring technologies have been shown to help clinicians safely and effectively monitor patients in the OR and medical-surgical floors," said Dr. Steve Barker, M.D., Ph.D., Chairman of the Scientific Advisory Board of Masimo. "Patient SafetyNet and SpHb, in particular, can equip caregivers with the right tools to meet new CMS guidance to improve patient safety and outcomes, while also reducing the cost of care."
1 Centers for Medicare & Medicaid Services. Survey & Certification Letter 14-15-Hospital. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Policy-and-Memos-to-States-and-Regions-Items/Survey-and-Cert-Letter-14-15
2 Draft National Action Plan for Adverse Drug Event (ADE) Prevention (2013). U.S. Department of Health and Human Services.
3 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. https://www.apsf.org/article/postoperative-monitoring-the-dartmouth-experience/
4 Vila H Jr, Smith RA, Augustyniak MJ: The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: Is patient safety compromised by treatment based solely on numerical pain ratings? Anesthesia and Analgesia, 2005;101:474-80
5 Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. Morbidity and Mortality Weekly Report 2010, 59:705-709
6 Office of Applied Studies, Substance Abuse and Mental Health Services Administration. Substance abuse treatment admissions involving abuse of pain relievers: 1998 and 2008,http://oas.samhsa.gov (accessed October 28, 2011)
7 McPherson ML: Strategies for the management of opioid-induced adverse effects. Advanced Studies in Pharmacy, 2008;5(2):52-57
8 Jarzyna D, et al: American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing, 2011;12(3):118-145.e10
9 Pasero C, M McCaffery: Pain assessment and pharmacologic management. Chapter 12 - Key Concepts in Analgesic Therapy, and Chapter 19 - Management of opioid-induced adverse effects. St. Louis, Mosby Elseveir, 2011
10 Joint Commission Issues New Sentinel Event Alert: Safe Use of Opioids in Hospitals, Aug. 8, 2012; http://www.jointcommission.org/sentinel_event_alert_safe_use_of_opioids_in_hospitals/
11 Awada W.F.N., Maher F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ. http://www.masimo.com/pdf/clinical/hemoglobin/Wael-STA-2013.pdf.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including total hemoglobin (SpHb), contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.


New Study Shows Masimo SET® Pulse Oximetry Helps Yale-New Haven Children's Hospital Significantly Reduce Retinopathy of Prematurity in Newborns
Irvine, California, May 15, 2014 – Masimo (NASDAQ: MASI) today announced that a new study published in the Journal of Perinatology showed that after the neonatal intensive care unit (NICU) at Yale-New Haven Children's Hospital switched to Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, there was a 37% lower rate of severe retinopathy of prematurity (ROP), an eye disorder that can cause blindness in premature babies, and a 53% lower rate of ROP requiring surgery.1
According to the study, "In 2006, an internal review of NICU-specific outcomes revealed that rates of BPD and severe ROP in our VLBW population were above the 50th percentile for pooled data from level III and IV NICUs. These data combined with several published reports outlining safe and successful efforts aimed at reducing (bronchopulmonary dysplasia) BPD and ROP through limiting hyperoxia2-4 resulted in the formation of the 'Oxidative Stress Initiative Committee.' The multidisciplinary committee was convened in January of 2007 and comprised physicians, neonatal nurse practitioners, physician assistants, registered nurses and respiratory therapists. A physician and nurse were identified to lead the initiative. Several steps were involved in this process including a detailed review of existing center-specific data and practices, discussion and analyses of relevant medical literature, creation of an evidence-based guideline, implementation of a new system for monitoring oxygen saturations and development and implementation of a unit-wide educational program."
In 2007, Yale-New Haven Children's Hospital launched an initiative to improve neonatal oxygen targeting. Researchers evaluated outcomes of newborns before and after the initiative. The pre-initiative period was Jan. 1, 2004 through Dec. 31, 2006. At the time, Yale-New Haven was using non-Masimo pulse oximeters in the NICU and an oxygen targeting range of 88% to 96%. The post-initiative period was Jan. 1, 2008 through Dec. 31, 2011, after Yale-New Haven had switched to Masimo SET® pulse oximetry "for its ability to more accurately measure SpO2 via algorithms and adaptive filters during episodes of motion or sever hypoxia."5,6 The study also noted Masimo pulse oximeters "had the ability to generate histograms and temporal data, which proved vital" because it enabled researchers "to quantify, for set time intervals, the percentage of time each patient was spending within, above and below their target SpO2 range." In addition, the oxygen targeting range was revised to 85% to 93% with the goal of maintaining each infant within their desired range for at least 75% of the day, education was provided to staff members, and the approach to management of oxygen for newborns in the delivery room was also revised.
A total of 304 infants in the pre-initiative period were compared with 396 infants in the post-initiative period. The rates of severe ROP in the pre- vs. post-initiative period were 24.6% vs. 15.4% (p=0.006), a 37% reduction. The rates of severe ROP requiring surgery in the pre- vs. post-initiative period were 36.2% vs. 16.9% (p=0.0003), a 53% reduction. After adjusting for other factors, the post-initiative period was associated with significantly lower odds of severe ROP compared with the pre-initiative period (adjusted odds ratio 0.41, 95% confidence interval 0.24 vs. 0.72, P = 0.002); researchers reported similar findings for infants requiring corrective laser surgery (adjusted OR 0.31, 95% confidence interval 0.17 vs. 0.59, P = 0.0003).
"We were able to demonstrate a significant reduction in the incidence of severe ROP and ROP requiring laser surgery in our NICU that directly coincided with our effort to limit hyperoxia," researchers said. "We believe that introduction of Signal Extraction Technology was a major key to this success."
Lead researcher Dr. Matthew Bizzarro at the Division of Perinatal Medicine, Department of Pediatrics, Yale University School of Medicine, also said the histogram functionality of the Masimo devices allowed clinicians to objectively observe whether their patients were within their target oxygenation ranges over time. "It's a technology that's greatly improved the way we approach and treat these babies," Dr. Bizzarro said. "We find it an invaluable tool to better managing this patient population."
The Yale-Newhaven study results are similar to those reported by two other centers in a previously published study.7 In the prior study, two separate NICUs that were staffed with the same physicians and nurses simultaneously changed their neonatal oxygen targeting range, and one of the centers switched from Nellcor to Masimo SET® pulse oximetry. In the first phase of the study, there was no decrease in severe ROP at the center using Nellcor pulse oximetry but there was a 58% reduction in severe ROP at the center using Masimo SET®. In the second phase of the study, the center still using Nellcor pulse oximetry switched to Masimo SET® and experienced a 54% reduction in severe ROP.
Masimo SET® significantly reduces false alarms (specificity) and accurately detects true alarms (sensitivity)8,9 that can help clinicians identify a deteriorating patient. Most importantly, Masimo SET® pulse oximetry has been clinically proven to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)10 in neonates, screen newborns for critical congenital heart disease (CCHD),11,12 reduce ventilator weaning time and arterial blood gas measurements in the ICU,13 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.9 It is estimated that Masimo SET® helps clinicians monitor more than 100 million patients each year and is the primary pulse oximetry technology for eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014).
"We are very happy to see yet another study showing that when a hospital NICU switches to Masimo SET pulse oximetry, they significantly reduce severe ROP and ROP requiring surgery," said Dr. Augusto Sola, Vice President of Neonatal Medical Affairs for Masimo. "Severe ROP and ROP-related blindness due to excessive supplemental oxygen unnecessarily harms one of society's most vulnerable patient populations, and as this study shows, can be drastically curtailed with the proper use of the right technology."
In babies born at full gestation, the blood vessels in the eye are fully developed. However, babies born prematurely often have abnormal blood vessel development in the retina of the eye, called ROP. These premature babies also often have lungs that are not fully developed, requiring the administration of supplemental oxygen in the NICU. Clinicians monitor oxygen saturation levels in the blood (SpO2) with pulse oximetry to help titrate the supplemental oxygen levels up and down to try and avoid periods of not enough oxygen in the blood (hypoxemia), which can lead to higher mortality, and too much oxygen in the blood (hyperoxia), which can exacerbate ROP. If pulse oximeters cannot accurately measure SpO2 during conditions of motion and low perfusion, which are common in the NICU, suboptimal oxygen titration can occur.
- Bizzarro MJ, Li FY, Katz K, Shabanova V, Ehrenkranz RA, Bhandari V. Temporal quantification of oxygen saturation ranges: an effort to reduce hyperoxia in the neonatal intensive care unit. Journal of Perinatology (2014) 34, 33-38; doi:10.1038/jp.2013.122; published online 26 September 2013
- Deulofeut R, Critz A, Adams-Chapman I, Sola A. Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. J Perinatol 2006; 26: 700–705.
- Castillo A, Sola A, Baquero H, Neira F, Alvis R, Deulofeut R et al. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: is 85 to 93% an acceptable range? Pediatrics 2008; 121: 882–889.
- Vandveen DK, Mansfield TA, Eichenwald EC. Lower oxygen saturation alarm. Limits decrease the severity of retinopathy of prematurity. J AAPOS 2006; 10: 445–448.
- Barker SJ. Motion-resistant pulse oximetry: a comparison of new and old models. Anesth Analg 2002; 95: 967–972.
- Hay WW, Rodden DJ, Collins SM, Melara DL, Hale KA, Fashaw LM. Reliability of conventional and new pulse oximetry in neonatal patients. J Perinatol 2002; 22: 360–366.
- Castillo AR, Deulofeut R, Sola A. Clinical Practice and SpO2 Technology in the Prevention of ROP in VLBW Infants. Presented at Pediatric Academic Societies Annual Meeting May 5-8, 2007.
- Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers" .J Clin Anesth. 2012 Aug;24(5):385-91.
- Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx.
- Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
- de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
- Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
- Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET® provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
-
Media Contacts:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo to Present at Bank of America Merrill Lynch 2014 Health Care Conference
Irvine, California, May 8, 2014 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Bank of America Merrill Lynch 2014 Health Care Conference at the Encore at the Wynn Las Vegas on Thursday, May 15, 2014, at 12:40 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Investor Contact:
Eli Kammerman
(949) 297-7077
[email protected]
Media Contacts:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Largest-Ever Study of Newborn Screening for Congenital Heart Disease Again Shows Masimo SET® Pulse Oximetry as Effective Tool
While Non-Masimo SET Technologies Have Not Been Shown to Be Effective
Irvine, California, April 30, 2014 – Masimo (NASDAQ: MASI)) today announced that the largest-ever study of newborn screening for congenital heart disease (CHD) was published online by The Lancet. The study of 122,738 newborns – more than twice as large as any previous study of this type – was conducted using Masimo SET® Measure-through Motion and Low Perfusion™ Pulse Oximetry and showed that Masimo SET® pulse oximetry screening significantly increased the rate of CHD detection and is "feasible and reliable" for detecting major congenital heart disease.1
Congenital heart defects are a leading cause of infant death in high-income countries, and affect eight of 1,000 livebirths.2 Critical congenital heart disease (CCHD), defined as causing death or requiring invasive intervention in the neonatal period, occurs in one to two newborns per 1,000 livebirths.3 Without CCHD detection and intervention, serious injury or death is common.
Senior researcher Dr. Guo-ying Huang and fellow researchers from the Paediatric Heart Centre, Children's Hospital of Fudan University, and the Shanghai Key Laboratory of Birth Defects in Shanghai, China conducted the study at 18 Chinese hospitals in 2011 and 2012. Each of the 122,738 newborns received a clinical assessment and pulse oximetry screening using a Masimo SET® hand-held pulse oximeter (Rad-5v®) with a Masimo SET® sensor (LNOP YI or LNOP Inf-L).
Congenital disease was detected in 1,071 cases, 157 of which were critical and 330 were major. In CCHD, clinical assessment alone had a sensitivity of 77.4% and pulse oximetry alone had a sensitivity of 83.6%. Compared to clinical assessment alone, Masimo SET® pulse oximetry screening increased the detection of CCHD from 77.4% to 93.2% and CHD from 81.3% to 90.2%. The false-positive rate for detection of critical disease was 2.7% (3,298 of 120,392) for clinical assessment alone and 0.3% (394 of 120,561) for pulse oximetry alone.
"Our findings showed that pulse oximetry alone had enough sensitivity and high specificity for detection of critical congenital heart disease," researchers stated. "Without pulse oximetry, discharge of asymptomatic newborn babies with undiagnosed congenital heart disease was three times more likely in babies with critical disease and almost twice as likely in babies with major disease. With combined pulse oximetry and clinical assessment, we detected 284 of 315 (90%) cases of critical congenital heart disease and 136 of 146 (93%) cases of major congenital heart disease." The researchers concluded: "This simple and accurate combined method should be used in maternity hospitals to screen for congenital heart disease."
The Lancet also published a commentary by renowned pediatrician Dr. Andrew K. Ewer, M.D., who said, "These findings would seem to put to rest any remaining concerns about accuracy, and therefore, clinical applicability of pulse oximetry screening." He noted that pulse oximetry screening also is useful in helping detect other disorders such as pneumonia and early onset sepsis, which "might be as lethal as critical congenital heart defect if not diagnosed in a timely manner."
In 2011, a U.S.-based CCHD workgroup recommended that all newborns be screened with "motion-tolerant pulse oximeters" that "have been validated in low-perfusion conditions." The workgroup relied upon two independent, published studies of nearly 60,000 subjects,4,5 which like the present study, also exclusively used Masimo SET® pulse oximetry. Masimo invented Measure-through Motion and Low Perfusion™ pulse oximetry and was the first company to get FDA clearance for accurate monitoring during motion and low perfusion. In 2011, the U.S. Department of Health and Human Services adopted the recommendations and added universal newborn screening by "motion-tolerant pulse oximeters" that "have been validated in low perfusion conditions" to the federal Recommended Uniform Screening Panel Guidelines.6 By the end of 2014, it is expected that 75% of U.S. states will require newborn screening for CCHD.7 However, in spite of the devastating impact of undetected CCHD and the strong evidence and recommendations, multiple U.S. states still do not require newborn screening for CCHD and no country other than the U.S. has adopted a national standard. In China alone, with 16 million annual births,8 there are up to 128,000 babies born with CHD and up to 32,000 born with CCHD each year – a significant portion of which are undetected today.
Because pulse oximetry is widely available in hospitals, many mistakenly assume that any pulse oximeter can be used for newborn screening. In fact, there are no large, appropriately powered newborn screening studies using non-Masimo pulse oximetry with similar effectiveness as the three large studies of over 180,000 newborns using Masimo SET®.
Masimo SET® pulse oximetry provides accurate measurements during motion and low perfusion based on a revolutionary technology that utilizes multiple algorithms that run in parallel to extract the true arterial signal from the composite signal (arterial and venous blood signals) that occurs during motion and low perfusion.
Dr. Anne Granelli – lead author on one of the largest published CCHD studies,5 liaison to the HHS workgroup, and author of an award-winning doctoral thesis in this field – has stated: "While working at one of the two centers in Sweden that performed pediatric cardiac surgery, I found a significant difference, when compared simultaneously with blood gas, between Masimo SET and all other pulse oximetry technologies we used on cyanotic children in the PICU and pediatric cardiac ward. We were not aware of the significant difference between oximeters before (our) clinical study. And, as a result, we upgraded all our pulse oximeters to Masimo SET."
"As we celebrate our 25th anniversary, we can't help but reflect upon what our technology has meant to tens of thousands of newborns and neonates who survive CCHD or no longer get Retinopathy of Prematurity. We are so happy to see yet another large study that shows what Masimo SET technology means to babies, their families and the clinicians who care for them around the globe," said Masimo founder and CEO Joe Kiani.
- Zhao Q-m, Ma X-j, Ge X-l, Liu F, Yan W-l, Wu L, Ye M, Liang X-c, Zhang J, Gao Y, Jia B, Huang G-y, Neonatal Congenital Heart Disease screening group. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. The Lancet, Early Online Publication, 23 April 2014. doi:10.1016/S0140-6736(14)60198-7
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011; 58: 2241–47.
- Chang RK, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med 2008; 162: 969–74.
- Ewer A.K., Middleton L.J. , Furmston A.T., Bhoyar A., Daniels J.P., Thangaratinam S., Deeks J.J, Khan K.S. Lancet. 2011 Aug 27;378(9793):785-94.
- de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
- Secretary of Health & Human Services letter to the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC); dated September 21, 2011.
- The Newborn Foundation, http://newborn-foundation.org
- Ministry of Health of the People's Republic of China. National birth defects control and prevention report (2012). Sept, 2012. http://www.gov.cn/gzdt/att/att/site1/20120912/1c6f6506c7f811bacf9301.pdf (accessed Oct 25, 2013).
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts: Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Celebrates 25th Anniversary of its Incorporation with Major Initiatives to Improve Patient Safety
Actions Include Day of Volunteering, $2.5 Million Donation and New Clinically Significant Product Announcements
Irvine, California, May 2, 2014 – Masimo (NASDAQ: MASI), the innovator of breakthrough patient monitoring technologies clinically shown to saves lives and the eyesight of newborns, and to help clinicians reduce unnecessary medical procedures and make better, faster decisions, today announced it will celebrate its Silver 25th Anniversary with major initiatives to improve patient safety.
Starting with a worldwide Day of Volunteering in the name of eliminating preventable patient deaths, teams of Masimo volunteers from across the globe will call on the world's acute-care hospitals to make public commitments to patient safety and report the measurable steps they have taken or will take to eliminate preventable patient deaths, aiding the Patient Safety Movement Foundation's goal of zero preventable deaths by 2020. The Day of Volunteering to eliminate preventable patient deaths is June 5, 2014.
More than 3 million preventable patient deaths occur each year worldwide, with more than 200,000 in U.S. hospitals.1 Masimo has been the presenting sponsor of the Patient Safety, Science & Technology Summit, which is the annual meeting of The Patient Safety Movement Foundation – a nonprofit 501(c) founded by Joe Kiani and the Masimo Foundation for Ethics, Innovation and Competition in Healthcare – dedicated to eliminating preventable patient deaths in U.S. hospitals by 2020.
Masimo volunteers seek to help the Patient Safety Movement Steering Committee enlist 1,000 hospitals. The hospitals that make public commitments will be announced at the third-annual Patient Safety, Science & Technology Summit in January 2015. At the conclusion of this year's Summit, the Patient Safety Movement announced that more than 60 hospitals and healthcare systems made commitments to help reduce preventable patient deaths, and at least one and 601 lives had been saved as a direct result of previous commitments.
As part of the 25th Anniversary, Masimo also announced the donation of $2.5 million to the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Foundation, founded in 2010, supports programs, initiatives, and research designed to improve patient safety and outcomes, promote efficient and cost-effective healthcare delivery, and provide advanced healthcare to people worldwide who may not otherwise have access to lifesaving technologies.
And in a long-planned move in anticipation of the 25th Anniversary, Masimo also will unveil at least one new clinically significant product a month for the remainder of this year, in an engineering push to accelerate innovative technologies that improve patient outcomes and safety, while reducing the cost of care.
These new products will build on the company's consumer product line, the Root® patient monitoring platform, and on revolutionary Masimo SET® Measure-through Motion and Low Perfusion pulse oximetry, the pioneering technology Masimo unveiled nearly two decades ago to solve the "unsolvable" problem of pulse oximetry's unreliability when it was needed most – during patient motion and low perfusion – as well as breakthrough rainbow® Pulse CO-Oximetry,™ allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), carboxyhemoglobin (SpCO®), and methemoglobin (SpMet®).
"We are humbled and honored to celebrate 25 years of medical technology innovation with the help of our dedicated global team and the support of our valued clinical and OEM partners," said Joe Kiani, founder and CEO of Masimo. "People have described us over the years as the company that puts patients first, the company that solves the unsolvable, the company that is not afraid of taking a stand for what is right, the company that has endured David vs Goliath obstacles, and even the 'Masters of Light.' But nothing means more to us than each and every story we hear of how we have helped save or improve the lives of individual patients and their families. Through our Day of Volunteering, the donation to the Masimo Foundation, and a planned release of new clinically relevant medical products, we are re-setting the bar even higher to help improve patient outcomes, not just through innovation, but what we can do through a higher social calling and action."
The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations. It is estimated that Masimo SET® helps clinicians monitor more than 100 million patients each year and is the primary pulse oximetry technology for eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014).
Compared to other pulse oximeters, during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms, and accurately detects true alarms2 that can indicate a deteriorating patient. ECRI Institute consistently ranks alarm management atop its annual Top 10 Technology Hazards list, noting that "caregivers can become overwhelmed trying to respond to the alarms, or they can become desensitized, which can lead to missed alarms or delayed response, placing patients at risk." Masimo SET® revolutionized pulse oximetry by reducing false alarms by 95% and improving the detection of true alarm conditions, not by long averaging or freezing, but by extracting the true signal in the presence of overwhelming noise.
Most importantly, Masimo SET® pulse oximetry has been shown to improve patient outcomes by enabling clinicians to reduce retinopathy of prematurity (ROP) in neonates,3 screen newborns for critical congenital heart disease (CCHD),4,5 reduce ventilator weaning time and arterial blood gas measurements in the ICU,6 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.7
"Since its introduction, it is estimated that Masimo SET pulse oximetry has prevented at least 25,000 potential cases of severe ROP and blindness in newborns worldwide, and has saved hundreds of thousands of lives of patients of all ages," said Dr. Augusto Sola, Vice President of Medical Affairs for Neonatology at Masimo. "Additionally in the past few years, Masimo SET pulse oximetry has saved from death or severe morbidities a significant number of infants with congenital heart disease and the number will grow as SET Pulse Oximetry screening is implemented more widely."
Meanwhile, Masimo rainbow® technology has been shown to make a clinical difference by improving patient safety and reducing cost of care. Noninvasive and continuous total hemoglobin (SpHb®), for example, has been shown to help clinicians reduce both the frequency of often-unnecessary multi-unit red blood cell (RBC) transfusions and the average number of RBC units transfused, and in cases where transfusions are needed, to help clinicians initiate transfusions faster.8
The American Medical Association and The Joint Commission have identified RBC transfusions, which can lead to higher mortality and infection rates,9 as one of the top five overused procedures in medicine.10
Another major noninvasive monitoring advancement has been SpCO®, shown in many independent clinical studies to be an effective front-line assessment tool to help clinicians identify more potential carbon monoxide (CO) poisonings – the leading cause of accidental poisoning deaths11 – more quickly, enabling faster intervention to improve patient outcomes.12,13,14,15
Joe Kiani, Concluded: "None of Masimo's achievements would have been possible without early believers in the company's vision. Special gratitude goes to the engineers, investors, as well as legal, healthcare and industry professionals who helped propel Masimo. Although too numerous to list here, a few are: Ammar Al-Ali, Steve Barker, Jim Bergman, Abbas Bolandgray, Anne de Wahl Granelli, Mohamed Diab, Robert Feibusch, Mitch Goldstein, Steve Jensen, Jack Lasersohn, Katsuyuki Miyasaka, Christian Poets, Joe Re, Larry Saper, Jim Scopa, Augusto Sola, Bob Smith, and Walt Weber. We thank them immensely!" A more comprehensive roster will be available in Masimo's upcoming Annual Report and posted at www.masimo.com.
- http://www.ncbi.nlm.nih.gov/pubmed/23860193
- Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
- Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
- de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
- Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
- urbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
- Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2.
- Wael NA, Mahmoued F. Reduction in Red Blood Cell Transfusions during Neurosurgery with Noninvasive and Continuous Hemoglobin Monitoring. Proceedings of the Society for Technology in Anesthesia Annual Meeting ; 2013 Jan 9-12; Phoenix AZ.
- Marik PE.et.al. Crit Care Med. 2008;36(9):2667-74
- Joint Commission Perspectives. The Joint Commission Continues to Study Overuse Issues. Volume 32, Number 5, 2012: 4-8(5).
- Hampson N., Weaver L. "Carbon Monoxide Poisoning: A New Incidence for an Old Disease." Undersea Hyperb Med 2007;34:163-168.
- Zorbalar N, Yesilaras M, Aksay E. "Carbon monoxide poisoning in patients presenting to the emergency department with a headache in winter months." Emerg Med J Published online ahead of print Oct. 15, 2013
- Sebbane M, Claret PG, Mercier G, Lefebvre S, Thery R, Dumont R, et al. "Emergency Department Management of Suspected Carbon Monoxide Poisoning: Role of Pulse Co-Oximetry." Respir Care. Published online ahead of print Mar 19, 2013.
- Suner S, Partridge R, Sucov A, et al. "Noninvasive Pulse CO-Oximetry Screening in the Emergency Department Identifies Occult Carbon Monoxide Toxicity." J. Emerg Med. 2008; 34(4): 441-450.
- Hampson N. "Noninvasive pulse CO-oximetry expedites evaluation and management of patients with carbon monoxide poisoning." The American Journal of Emergency Medicine 2012 (10.1016/j.ajem.2012.03.026)
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including total hemoglobin (SpHb), contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions with comparable accuracy and unique advantages, including: continuous results that enable earlier treatment without causing invasive trauma in all patients and in every clinical situation; risks related to our belief that Masimo SpCO will provide an accurate and effective noninvasive method of screening for CO poisoning; risks related to our belief that SpCO offers an effective front-line assessment tool; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


Masimo Reports First Quarter 2014 Financial Results
Q1 2014 Highlights (compared to Q1 2013):
- Product revenue rose 3% to $132.2 million, net of a $2.6 million current quarter adjustment to our deferred revenue estimate for a certain U.S. distributor; absent this adjustment, product revenue rose 5% to $134.8 million
- Total revenue, including royalties, rose 3% to $139.8 million; absent the same $2.6 million adjustment, total revenue, including royalties, rose 5% to $142.4 million
- Masimo rainbow revenue rose 23% to $12.9 million
- SET® and rainbow® SET® unit shipments were 41,400
- Earnings per share rose 39% to $0.39 including a $0.09 gain related to an $8.0 million reversal of a prior period accrual
Irvine, California, April 30, 2014 – Masimo (NASDAQ: MASI) today announced its financial results for the first quarter ended March 29, 2014.
First quarter 2014 GAAP product revenues and total revenues, including royalties, were $132.2 million and $139.8 million, respectively, both increasing 3% over the prior year first quarter. Included within such revenues for the quarter was a $2.6 million adjustment related to a revision for deferred revenue related to inventory on-hand at one US distributor. Without this adjustment, first quarter 2014 product revenues rose 5% to $134.8 million, compared to $128.6 million for the first quarter of fiscal year 2013, and total revenue, including royalties, rose 5% to $142.4 million, up from $135.9 million for the first quarter of fiscal year 2013. The company's worldwide direct product revenue in the first quarter of 2014 rose by 3%, or 5% without the adjustment, compared to the same period in 2013 and represented 84% of product revenue with and without the adjustment. OEM sales, which accounted for 16% of product revenue, rose by 2% compared to the same period in 2013. Revenue from sales of Masimo rainbow products rose 23% to $12.9 million in the first quarter of 2014, compared to $10.5 million in the year-ago period.
Net income for the first quarter of 2014 was $22.6 million, or $0.39 per diluted share, compared to net income of $16.4 million, or $0.28 per diluted share, in the first quarter of 2013. Net income for the first quarter of 2014 included the reversal of a Q4 2013 arbitration and related legal expense accrual in the amount of $8.0 million, or $0.09 per share.
During the first quarter of 2014, the company shipped approximately 41,400 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units, an increase of 5% compared to the same prior-year period. Masimo estimates its worldwide installed base as of March 29, 2014 to be 1,231,000 units, up 10% from 1,117,000 units as of March 30, 2013.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo began 2014 with continuing gains in market share and a 10% increase in our global installed base. In conjunction, with our 25th anniversary celebration, we plan to announce multiple new products in 2014, including innovative devices which will continue to help improve patient care and patient safety, while reducing cost of care. Our outlook remains positive as we drive towards our mission and financial objectives."
As of March 29, 2014, Masimo's cash and cash equivalents were $117.5 million, compared to $95.5 million as of December 28, 2013. The change reflects primarily net cash generated from operations.
2014 Financial Guidance
Masimo today is providing updated 2014 financial guidance. Masimo now expects fiscal 2014 total revenue to be approximately $588 million to $598 million, including product revenue of approximately $560 million to $570 million and royalty revenue of approximately $28 million. In addition, Masimo now expects fiscal 2014 GAAP earnings per share to range between $1.24 and $1.33. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live webcast of the call will be For further reading visit: from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 31415337. After the live webcast, the call will be available on Masimo's website through May 30, 2014. In addition, a telephonic replay of the call will be available through May 14, 2014. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 31415337.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our expectations for full fiscal year 2014 total, product, rainbow and royalty revenues and GAAP earnings per share; our plan to announce multiple new products in 2014; our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET® and Masimo rainbow® SET® products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the amount and type of equity awards that we may grant to employees and service providers in the future; our ongoing litigation and related matters; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
-
Investor Contact: Eli Kammerman
(949) 297-7077
[email protected] -
Media Contacts: Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation
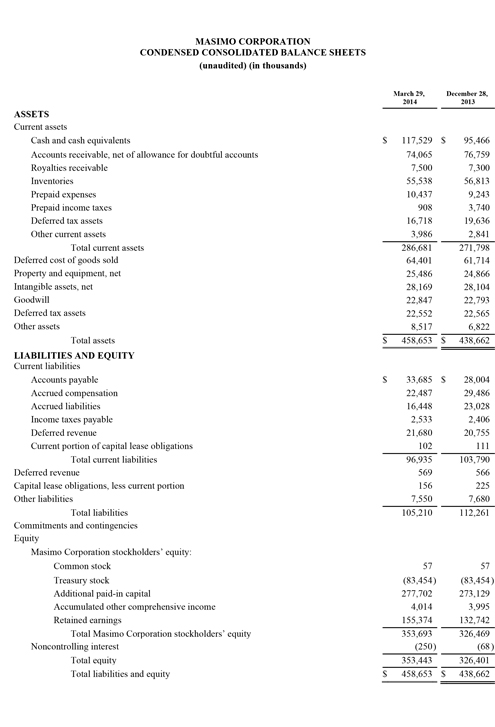
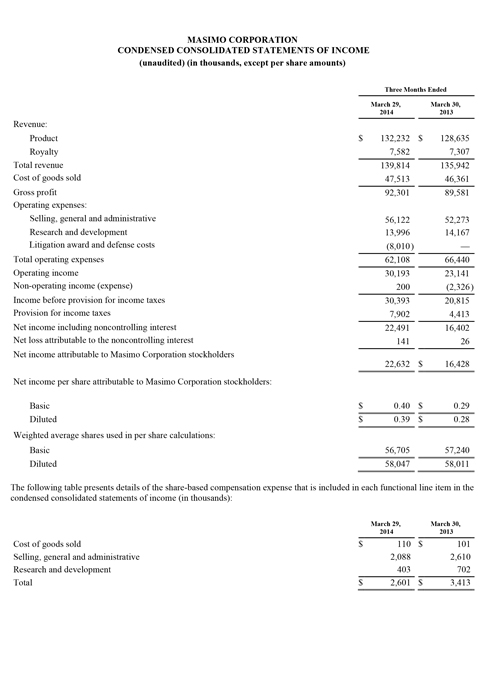
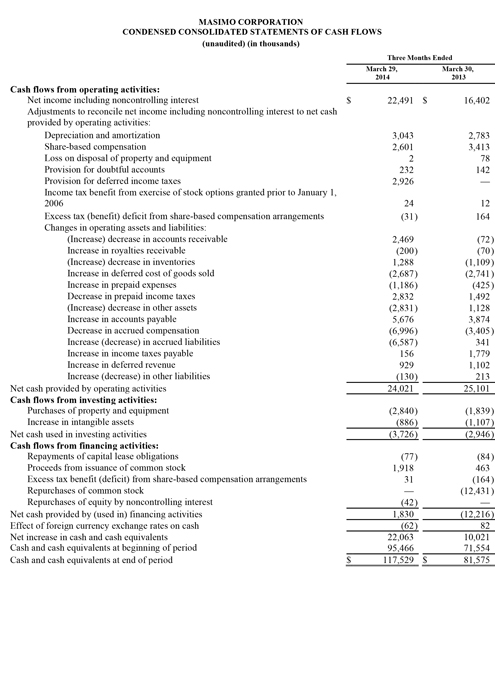


Masimo to Report First Quarter 2014 Financial Results after Market Close on Wednesday, April 30
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., April 17, 2014 – Masimo (NASDAQ: MASI) announced today that it will release financial results for the first quarter ended March 29, 2014, after the market closes on Wednesday, April 30, 2014. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be For further reading visit: from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 31415337. After the live webcast, the call will be available on Masimo's website through May 30, 2014. In addition, a telephonic replay of the call will be available through May 14, 2014. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 31415337.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
-
Investor Contact:
Eli Kammerman
(949) 297-7077
Masimo Corporation
[email protected] -
Media Contacts:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation


New Clinical Study Shows Masimo PVI® Helps Clinicians Optimize Fluid Administration in Patients Under Combined General and Epidural Anesthesia
Irvine, California – April 7, 2014 – Masimo (NASDAQ: MASI) announced today a recent study in the Journal of Monitoring and Computing demonstrates that Masimo's noninvasive PVI® parameter can help clinicians optimize fluid management and reduce unnecessary fluid administration for abdominal surgical patients under combined general and epidural anesthesia.1
Combining general and epidural anesthesia reduces the need for general anesthetics and can reduce risks of respiratory complications and time of stay in hospitals, but the occurrence and severity of hypotension (low blood pressure) are higher compared to general anesthesia alone without proper fluid management.2-4 Too little fluid administration can result in low perfusion in peripheral tissue, but too much fluid administration can result in fluid overload postoperatively.5,6 Traditional management approaches to guide fluid administration including central venous pressure (CVP) monitoring are not considered reliable.7 Instead of so-called "static" parameters such as CVP, experts recommend the use of "dynamic" parameters that measure variations over the respiratory cycle. Multiple dynamic parameters have been shown to help clinicians predict fluid responsiveness and improve fluid management, but most dynamic parameters require invasive and/or complex methods. In contrast, PVI is noninvasive and easily obtained with any Masimo SET® or rainbow® sensor. PVI provides clinicians with a continuous, easy-to-use, and cost-effective measure for assessing whether patients will benefit from fluid administration – enabling personalized and goal-directed fluid therapy.8-12
In the study conducted at Shanghai First People's Hospital in Shanghai, China, Yinan Yu, M.D., and a team of researchers assessed the impact of PVI as a goal-directed fluid parameter on tissue perfusion for 30 patients who underwent elective abdominal surgery while administered combined general and epidural anesthesia (GEN-EPI). Investigators used a Masimo Radical-7 rainbow SET Pulse CO-Oximeter®, which automatically calculated and displayed PVI.
The total average amount of fluid infused in PVI-guided fluid therapy group was significantly lower than the amount of fluid in the control group (1,918 ± 437 mL vs. 2,327 ± 463 mL, P < 0.05). The total amount of crystalloid fluid received in the PVI group also was greatly less than that of the control group (1,355 ± 383 mL vs. 1,715 ± 406 mL, P < 0.05). Moreover, there were significantly lower blood lactate levels during the first hour of the surgery between the PVI group (0.81 ± 0.30 mmol//L) and the control group (1.18 ± 0.32 mmol/L), P < 0.05.
The researchers noted that higher blood lactate levels in the perioperative period recently have been shown to be related with postoperative complications and the average hospitalization time.13
The researchers concluded: "PVI-based goal-directed fluid management in major abdominal surgery patients under GEN–EPI can reduce the amount of fluids administration, especially the crystalloid administration, and the blood lactate levels during surgery. Furthermore, the first hour following GEN–EPI is the critical period for anesthesiologists to optimize the fluid therapy."
Steve Barker, M.D., Ph.D., Chairman of the Scientific Advisory Board of Masimo, stated: "This study demonstrates the clinical advantage of PVI in optimizing fluid management and reducing unnecessary fluid administration for a significant population of patients, and adds to the growing body of evidence that shows how PVI – by aiding clinicians in understanding fluid levels of their patients – can improve patient outcomes while helping to reduce costs."
1 Yu Y, Dong J, Xu Z, Shen H, Zheng J. "Pleth variability index-directed fluid management in abdominal surgery under combined general and epidural anesthesia." J Clin Monit Comput 2014 Feb; DOI 10.1007/s10877-014-9567-5. For further reading visit: http://link.springer.com/article/10.1007/s10877-014-9567-5.
2 Zhao W, Zhou R, Zhou LP, Li CH. The hemodynamic effects during thoracic epidural anesthesia combined with general anesthesia in patients undergoing major abdominal operations. Zhonghua wai ke za zhi [Chin J Surg]. 2009;47(11):849–52.
3 Gurses E, Berk D, Sungurtekin H, Mete A, Serin S. Effects of high thoracic epidural anesthesia on mixed venous oxygen saturation in coronary artery bypass grafting surgery. Med Sci Monit Int Med J Exp Clin Res. 2013;19:222–9. doi:10.12659/MSM.883861.
4 Shin S, Bai SJ, Rha KH, So Y, Oh YJ. The effects of combined epidural and general anesthesia on the autonomic nervous system and bioavailability of nitric oxide in patients undergoing laparoscopic pelvic surgery. Surg Endosc. 2013;27(3):918–26. doi:10. 1007/s00464-012-2536-5.
5 Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth. 2002;14(4):252–6.
6 Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid T. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8. doi:10.1097/01.sla.0000094387.50865.23.
7 Vallet B., Blanloeil Y., Cholley B., Orliaguet G., Pierre S., Tavernier B. "Strategy for perioperative vascular filling - Guidelines for perioperative haemodynamic optimization." Experts' Formalized Recommendations, French Society of Anaesthesia and Intensive Care (SFAR), Validation by the administrative council of SFAR on 19 October 2012.
https://pubmed.ncbi.nlm.nih.gov/24126197/.
8 Loupec T., Nandoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene B., Dahyot-Fizelier C., Mimoz O. "Pleth Variability Index Predicts Fluid Responsiveness in Critically-Ill Patients." Crit Care Med 2011 Feb;39(2):294-9. For further reading visit: http://journals.lww.com/ccmjournal/Abstract/2011/02000/Pleth_variability_index_predicts_fluid.8.aspx.
9 Zimmerman M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B., and Wiesenack C. "Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to Predict Fluid Responsiveness in Mechanically-ventilated Patients Undergoing Major Surgery." Eur J Anaesthesiol. 2010 Jun;27(6):555-61. For further reading visit: http://www.ncbi.nlm.nih.gov/pubmed/20035228.
10 Feissel M., Kalakhy R., Badie J., Robles G., Faller J., Teboul JL. "Plethysmography Variability Index: A New Fluid Responsiveness Parameter." Presented at the 29th International Symposium on Intensive Care and Emergency Medicine (ISICEM) Annual Meeting, March 25, 2009, Brussels, Belgium. For further reading visit: http://ccforum.com/content/13/S1/P205.
11 Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot JJ. "Pleth variability Index to Monitor the Respiratory Variations in the Pulse Oximeter Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the Operating Theatre." Br J Anaesth. 2008 Aug;101(2):200-6. For further reading visit: http://www.ncbi.nlm.nih.gov/pubmed/18522935.
12 Forget P, Lois F, De Kock M. "Goal-Directed Fluid Management Based on the Pulse Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid Management." Anesth Analg. 2010 Oct;111(4):910-4.
Published online at http://www.anesthesia-analgesia.org/content/early/2010/08/12/ANE.0b013e3181eb624f.abstract?ct=ct.
13 Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi:10.1186/2110-5820-3-12.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com .
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions of the repeatability of clinical results obtained using Masimo PVI, risks related to our belief that PVI is an easy-to-use and cost-effective measure for assessing whether patients will benefit from fluid administration, risks related to our assumptions that PVI enables personalized and goal-directed fluid therapy, and that PVI is a preferred noninvasive indicator of fluid responsiveness in children, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation.


Masimo Expands Its Board of Directors with Appointment of Medical Device Executive Craig Reynold
Irvine, California – April 4, 2014 – Masimo (NASDAQ: MASI), a global innovator of noninvasive patient monitoring technologies, today announced the appointment of seasoned medical device executive Craig B. Reynolds to the Board of Directors of Masimo. Reynolds also will serve as a member of the board's Audit Committee as well as the Compensation Committee.
"Masimo has been at the forefront of medical technology innovation, patient safety, and cost efficiencies in hospital environments – and has done so with ethics and integrity," Reynolds said. "I look forward to joining a truly impressive Board of Directors and am confident that my medical device experience will allow me to contribute to Masimo's continued success."
Reynolds, 65, has served as a director of Symmetry Medical, Inc. (NYSE: SMA) since Jan. 4, 2008, and is currently Chairman of the Board. He is the Chief Executive Officer and a Director of Cereve, Inc., a medical company engaged in resolving insomnia. Prior to joining Cereve, Reynolds served as Chief Operating Officer of Philips Respironics Home Health Solutions, a subsidiary of Philips, from 2008 to 2010. Prior to Philips Respironics, Reynolds was the Chief Operating Officer and a Board Member of Respironics, Inc. (NASDAQ: RESP), a company that develops, manufactures and markets medical devices worldwide. Reynolds also served as CEO and Director of medical device company Healthdyne Technologies, Inc. (NASDAQ: HDTC).
He earned his B.S. in industrial management from the Georgia Institute of Technology and his M.B.A. from Georgia State University.
"We are honored to have a professional of Craig's caliber on our team," said Masimo Chairman and CEO, Joe Kiani. "Craig brings a unique combination of Executive and Board experience from the medical technology sector as well as a long track record of doing the right things the right way. As a result, I am confident that he will be a valuable member of our board."
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and PVI®, in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com .
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo Corporation

![]()

Flagler Hospital Upgrades to Masimo SET® Pulse Oximetry for Improved Patient Outcomes
St. Augustine, Florida & Irvine, California – March 6, 2014 – Masimo (NASDAQ: MASI) today announced that Flagler Hospital – ranked among the top 5% of all hospitals in the nation for both clinical excellence and patient safety for the past eight consecutive years – has upgraded system-wide to Masimo SET® pulse oximetry, the standard-of-care at leading hospitals around the world.
Flagler Hospital joins a growing and distinguished roster of health organizations using Masimo SET® pulse oximetry, clinically shown to virtually eliminate false alarms1 and help clinicians detect life-threatening events.2 Flagler Hospital's standardization to Masimo SET® pulse oximetry is in keeping with the healthcare organization's dedication to excellence in care and delivering the best possible results for its patients.
"Since Flagler Hospital began using Masimo pulse oximeters, we have seen quite a difference in the level of performance," said Jim Clifton, BioMed Supervisor at Flagler Hospital. "We have far fewer nuisance alarms than before, and we are able to obtain accurate readings, even when patients are moving or have low perfusion."
The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations. Masimo SET® is trusted by clinicians to safely monitor more than 100 million patients each year and is used hospital-wide by eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014). Compared to other pulse oximeters, during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)1 that can indicate a deteriorating patient. Most important, Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)3 in neonates, screen newborns for critical congenital heart disease (CCHD),4,5 reduce ventilator weaning time and arterial blood gas measurements in the ICU,6 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.7
"We are honored to partner with Flagler Hospital, which has a well-deserved reputation for having patient empathy and providing quality care," said Masimo Founder and CEO Joe Kiani. "We also appreciate and share Flagler Hospital's commitment to excellence and look forward to helping this great hospital and staff meet and exceed their patient-care needs."
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: http://journals.lww.com/anesthesiology/Fulltext/2010/02000/Impact_of_Pulse_Oximetry_Surveillance_on_Rescue.10.aspx.
3 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
4 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
5 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
6 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
7 Taenzer A, et al. Anesthesiology, 2010 Feb;112(2):282-7.
About Flagler Hospital
Flagler Hospital is a 335 bed, acute care hospital that has been ranked among the top 5% of all hospitals in the nation for both clinical excellence and patient safety for the past eight consecutive years. The hospital has operated as a not-for-profit healthcare institution in St. Augustine, Florida since its founding in 1889. Flagler Hospital's focus on quality has resulted in numerous national accreditations including, designation as a Chest Pain Center by the Society of Chest Pain Centers, ANCC Magnet Status for Nursing Excellence, the Gold Seal of Approval™ from The Joint Commission for Primary Stroke Care Centers, National accreditation for its total hip and total knee replacement programs, accreditation of its Cancer Institute by the Commission on Cancer and ASMBS Center of Excellence Designation for its Bariatric Surgery Center. To learn more about all of the hospital's services log on to www.FlaglerHospital.org.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care-helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that the hospital-wide conversion ensures that all Flagler Hospital patients will be cared for using the most technologically and clinically advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Emily Stimler
Flagler Hospital
Phone: (904) 819-4669
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care... by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN is under license from University HealthSystem Consortium.

Masimo to Present at 26th Annual ROTH Conference
Irvine, California, February 28, 2014 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 26th Annual ROTH Conference at the Ritz-Carlton, Laguna Niguel on Tuesday, March 11, 2014, at 10:00 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.



North Oaks Medical Center Is First to Integrate Masimo Patient SafetyNet™ with EPIC Electronic Medical Record System
Hammond, Louisiana & Irvine, California – February 25, 2014 – Masimo (NASDAQ: MASI) and North Oaks Medical Center – the 330-bed flagship of North Oaks Health System – today announced that the facility has gone live with a two-way interface between its EPIC electronic medical record and the Masimo Patient SafetyNet™ remote monitoring and clinician notification system.
The installation marks the first time a hospital has integrated Patient SafetyNet with the Epic electronic medical record (EMR) system. This integration supports the hospital's efforts to achieve Meaningful Use objectives while simplifying nursing workflow, and enabling near real-time availability of patient data within the Epic EMR, supporting clinical decision making processes and eliminating errors associated with manual charting.
Patient SafetyNet incorporates the Masimo Adaptive Connectivity Engine™ (ACE), which enables two-way, HL7-based connectivity to the EMR. ACE significantly reduces the time and complexity to integrate and validate custom HL7 implementations. Patient SafetyNet has been clinically shown to keep patients safer by enabling remote continuous monitoring of the patient's physiology, including oxygenation and pulse rate, which has led to saved lives, reductions in rapid response activations and transfers to intensive care units.1
Integrating Patient SafetyNet with the EMR achieves the type of medical technology interoperability – the ability of medical devices and health care systems to seamlessly communicate and exchange information – called for by leading health organizations to improve patient outcomes and reduce costs.
"We are excited to have integrated Masimo with our electronic health record and look forward to using Patient SafetyNet as another tool to assist in patient care," explains Herbert Robinson, MD, North Oaks Health System's Chief Medical Information/Informatics Officer.
"Medical device interoperability is a key component to improving clinical decision making, patient safety, and clinician workflow. Unfortunately many technical and corporate barriers can make achieving interoperability challenging for hospitals," said Joe Kiani founder and CEO of Masimo.
"Our goal is to eliminate these barriers, and the Adaptive Connective Engine demonstrates Masimo's commitment to innovation that automates patient care with open, scalable, and standards-based connectivity architecture. We are excited to have partnered with North Oaks Medical Center to achieve the integration of the Patient SafetyNet system with their Epic EMR, and to support them in achieving their interoperability goals."
1 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. https://www.northoaks.org/
About North Oaks Health System
North Oaks Health System is one of Louisiana's largest and most progressive community hospital organizations and is strategically based between New Orleans and Baton Rouge. For more than 50 years, we have made it our mission to optimize the health care experience through compassion and innovation. Facilities in Tangipahoa and Livingston Parishes include an acute care hospital, a rehabilitation hospital, two outpatient diagnostic & treatment centers, an outpatient surgery center, two outpatient rehabilitation clinics, a hospice agency and a growing physician group for primary and specialty care. For more information, please visit www.northoaks.org
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions that all patients at North Oaks Medical Center will be cared for using the most technologically and clinically-advanced noninvasive patient monitoring solutions available; risks related to our belief that Masimo Patient SafetyNet can help keep patients safer by noninvasively, continuously measuring and tracking their underlying physiological condition to help hospitals avoid preventable patient deaths and injuries associated with failure to rescue events; risks related to our belief that Masimo SET virtually eliminates false alarms and increases a clinician's ability to detect life-threatening events; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these forward-looking statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Melanie Lanaux Zaffuto
North Oaks Health System
Phone: (985) 230-6555
Email: [email protected]
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium. All other trademarks, service marks, trade names, logos and icons, registered or not, are the property of third parties as named/indicated.


Masimo to Present at Raymond James 35th Annual Institutional Investors Conference
Irvine, California, February 21, 2014 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the Raymond James 35th Annual Institutional Investors Conference at the JW Marriott Grande Lakes Orlando on Tuesday, March 4, 2014, at 7:30 a.m. Eastern Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
# # #
Investor Contact: Eli Kammerman
(949) 297-7077
[email protected]
Media Contacts: Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.



Masimo Unveils iSpO2® Pulse Oximeter for Android™
Irvine, California – February 20, 2014 – Masimo (NASDAQ: MASI) today announced that its award-winning iSpO2® pulse oximeter is now available for Android™, along with the companion iSpO2 app downloaded from the Google Play™ Store. With the release of iSpO2 in the popular Android operating system, more consumers than ever now have access to Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry – the same technology used in leading hospitals worldwide.

iSpO2® for Android provides oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI).
iSpO2 provides accurate, real-time oxygen saturation (SpO2), pulse rate (PR), and perfusion index (PI) readings – ideal for anyone who desires access to accurate health data through their mobile devices.
Stig Severinsen, Ph.D. in medicine, a four-time World Champion freediver and owner of multiple Guinness World Records, including history's longest breath-hold of 22 minutes, uses iSpO2 as part of his regimen.
"Training for extreme records under extreme conditions is always a huge challenge," Severinsen said. "In such situations it is of great value to be able to perform noninvasive and accurate measurements of my heart rate and oxygen levels with a state-of-the-art device. Masimo has created a clever device that not only displays your measurements in real time – even during motion and states of low perfusion in cold conditions – but it also allows you to store and share data with people in your team.
"I would recommend Masimo's iSpO2 to anyone interested in health and fitness – understanding what goes on inside your body is paramount to improving performance," he added.
Dr. Mark Hom, M.D., co-author of forthcoming exercise books with three-time Tour de France champion Greg LeMond, and Dr. Glenn A. Gaesser, Ph.D., said: "Although we advocate intense training, we don't recommend that healthy athletes train to the point of oxygen desaturation (i.e., cyanosis). However, our preliminary testing with the iSpO2 has shown that it has the accuracy and repeatability to show subtle changes in athletic oxygenation: specifically in warm-up, steady state, super high intensity, and recovery. And as an added benefit, the iSpO2 is compact enough to be useful when training on the road or track"
"This pulse oximeter is without a doubt the best one available for the consumer market," said Dr. Kirk Shelley, M.D., PhD, professor of anesthesiology at Yale University in New Haven, Conn. "Masimo uses impressive digital signal processing combined with proprietary light emitting diode (LED) technology. If you need a serious pulse oximeter, this is the one to get."
iSpO2 – extremely lightweight at just 232 grams or about 0.5 pounds – also displays the pleth waveform and Signal IQ™ so users can visually assess where the pulse is occurring and the quality of the measurement even during motion artifact. iSpO2 can also trend, store, and email up to 12 hours of measurement history in the global standard, .CSV file format, allowing consumers to easily share data through their mobile device email application.
The performance of Masimo SET® pulse oximetry is proven by more than 100 independent and objective studies and thousands of clinical evaluations to accurately monitor blood oxygen saturation even in the most challenging conditions, including patient movement and low peripheral perfusion. The pulse oximetry standard-of-care at leading hospitals worldwide – including by eight of the top 10 hospitals on the U.S. News & World Report Best Hospitals Honor Roll (2013-2014) – Masimo SET® virtually eliminates false alarms1 and increases a clinician's ability to detect life-threatening events.2
Android is a trademark of Google Inc. The Android robot is reproduced or modified from work created and shared by Google and used according to terms described in the Creative Commons 3.0 Attribution License.
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: https://pubs.asahq.org/anesthesiology/article/132/3/602/108898/Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors related to the iSpO2, including our belief in the breakthrough ability of Masimo SET® pulse oximetry to measure-through motion and low perfusion; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SedLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium. All other trademarks, service marks, trade names, logos and icons, registered or not, are the property of third parties as named/indicated.

Masimo to Present at 2014 Citi Global Healthcare Conference
Irvine, California, February 18, 2014 – Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 2014 Citi Global Healthcare Conference at the New York Hilton Midtown on Tuesday, February 25, 2014, at 10:00 a.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.


Masimo Reports Fourth Quarter and Full Year 2013 Financial Results
Q4 2013 Highlights (compared to Q4 2012):
- Total revenue, including royalties, rose 8% to $142.4 million
- Product revenue rose 8% to $134.7 million
- Masimo rainbow revenue rose 34% to $14.8 million
- SET® and rainbow® SET® unit shipments were 42,000
- GAAP earnings per share was $0.16 including charges of $0.15 per share
Full Year 2013 Highlights (compared to 2012):
- Total revenue, including royalties, rose 11% to $547.2 million
- Product revenue rose 11% to $517.4 million
- Masimo rainbow revenue rose 21% to $48.8 million
- Shipped 168K Masimo SET® and rainbow® SET® units
- GAAP earnings per share was $1.02, including Q4 charges of $0.15 per share
Irvine, California, February 13, 2014 – Masimo (NASDAQ: MASI) today announced its financial results for the fourth quarter and year ended December 28, 2013.
Masimo's total fourth quarter revenue, including royalties, rose 8% to $142.4 million, compared to $132.2 million for the fourth quarter of 2012. Fourth quarter 2013 product revenue rose 8% to $134.7 million, compared to $125.3 million for the fourth quarter of 2012. The company's worldwide direct product revenue grew 9% in the fourth quarter of 2013 and represented 86% of product revenue. OEM sales, which accounted for 14% of product revenue, declined by 1% compared to the same period in 2012. Revenue from sales of Masimo rainbow products rose 34% to $14.8 million in the fourth quarter, compared to $11.1 million in the year-ago period.
Net income for the fourth quarter of 2013 was $9.3 million, or $0.16 per diluted share, compared to net income of $15.0 million, or $0.26 per diluted share, in the fourth quarter of 2012. Net income for the fourth quarter of 2013 included pre-tax charges of $4.6 million related to selected inventory and equipment related write-downs related to accelerated technology transitions and $8.0 million in various charges related to the previously announced arbitration award ruling. Together, these combined charges reduced fourth quarter GAAP earnings per share by $0.15.
During the fourth quarter of 2013, the company shipped approximately 42,000 SET® pulse oximetry and rainbow® Pulse CO-Oximetry™ units, excluding handheld units, approximately level with the same prior-year period. Masimo estimates its worldwide installed base as of December 28, 2013 to be 1,205,000 units, up 11% from 1,088,000 units as of December 29, 2012.
Joe Kiani, Chairman and Chief Executive Officer of Masimo, said, "Masimo achieved many important objectives in 2013, including an 11% increase in our global installed base, increased adoption of pulse oximetry across the U.S. which helps clinicians screen for congenital heart disease in newborns, and the launch of Root™, which includes open architecture monitoring and connectivity features in CE Mark countries. We have begun 2014 with the CE Mark for O3™, our new tissue and cerebral oximetry device, and expect to announce other new products this year. In addition, we are confident that value engineering initiatives that we have put in place since 2012 will begin to result in increased product margin and improved product performance throughout 2014 and beyond."
As of December 28, 2013, Masimo's cash and cash equivalents were $95.5 million, compared to $71.6 million as of December 29, 2012. The change reflects primarily net cash generated from operations, offset by $19.8 million in cash used to repurchase one million shares of Masimo common stock in 2013.
2014 Financial Guidance
Masimo today is providing 2014 financial guidance. Masimo expects fiscal 2014 total revenues to be approximately $578 million to $598 million, including product revenues of $570 million and royalty revenues ranging between $8 million to $28 million. Included in the 2014 product revenue guidance is a rainbow revenue expectation of $60 million. In addition, Masimo expects fiscal 2014 GAAP earnings per share to range between $1.13 and $1.28. Each of the components of Masimo's guidance set forth above is an estimate only and actual performance could differ.
Conference Call
Masimo will hold a conference call today at 1:30 p.m. PT (4:30 p.m. ET) to discuss the results. A live
webcast of the call will be For further reading visit: from the investor relations page of the company's website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 54039233. After the live webcast, the call will be available on Masimo's website through March 15, 2014. In addition, a telephonic replay of the call will be available through February 27, 2014. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 54039233.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care ... by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements including, in particular, the statements about our financial condition, results of operations and business generally; expectations regarding our ability to design and deliver innovative new noninvasive technologies and reduce the cost of care; and demand for our technologies. These forward-looking statements are based on management's current expectations and beliefs and are subject to uncertainties and factors, all of which are difficult to predict and many of which are beyond our control and could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to, those related to: our dependence on Masimo SET and Masimo rainbow SET products and technologies for substantially all of our revenue; any failure in protecting our intellectual property exposure to competitors' assertions of intellectual property claims; the highly competitive nature of the markets in which we sell our products and technologies; any failure to continue developing innovative products and technologies; the lack of acceptance of any of our current or future products and technologies; obtaining regulatory approval of our current and future products and technologies; the risk that the implementation of our international realignment will not continue to produce anticipated operational and financial benefits, including a continued lower effective tax rate; the loss of our customers; the failure to retain and recruit senior management; product liability claims exposure; a failure to obtain expected returns from the amount of intangible assets we have recorded; the maintenance of our brand; the impact of the decline in the worldwide credit markets on us and our customers; the integration of acquisitions; the amount and type of equity awards that we may grant to employees and service providers in the future; and other factors discussed in the "Risk Factors" section of our most recent periodic reports filed with the Securities and Exchange Commission ("SEC"), including our most recent Form 10-K and Form 10-Q, all of which you may obtain for free on the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
# # #
Investor Contact: Eli Kammerman
(949) 297-7077
[email protected]
Media Contact: Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo to Report Fourth Quarter and Full Year 2013 Financial Results after Market Close on Thursday, February 13
Conference call and webcast to begin at 1:30 p.m. PT (4:30 p.m. ET)
IRVINE, Calif., January 30, 2014 -- Masimo (NASDAQ: MASI) announced today that it will release fourth quarter and full year 2013 financial results for the period ended December 28, 2013, after the market closes on Thursday, February 13, 2014. The conference call to review the results will begin at 1:30 p.m. PT (4:30 p.m. ET) and will be hosted by Joe Kiani, Chairman and Chief Executive Officer, and Mark P. de Raad, Executive Vice President and Chief Financial Officer.
A live webcast of the conference call will be For further reading visit: from the investor relations page of the company's corporate website at www.masimo.com. The dial-in numbers are (888) 520-7182 for domestic callers and +1 (706) 758-3929 for international callers. The reservation code for both dial-in numbers is 54039233. After the live webcast, the call will be available on Masimo's website through March 15, 2014. In addition, a telephonic replay of the call will be available through February 27, 2014. The replay dial-in numbers are (800) 585-8367 for domestic callers and +1 (855) 859-2056 for international callers. Please use reservation code 54039233.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
# # #
Contact:
Eli Kammerman
(949) 297-7077
[email protected]
Media Contact:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.


Masimo Announces CE Marking of O3™ Regional Oximetry
Study Presented at Society of Technology in Anesthesia Annual Meeting Demonstrates 4.0% Absolute Accuracy and 2.1% Trend Accuracy
Irvine, California – January 23, 2014 – Masimo (NASDAQ: MASI) announced today the CE Marking of O3™ regional oximetry for the Root™ patient monitoring and connectivity platform. O3 regional oximetry is a new technology developed by Masimo and uses near-infrared spectroscopy (NIRS) in a Masimo Open Connect (MOC-9™) module with up to two sensors per MOC-9 module. Each sensor contains four light-emitting diodes (LEDs) and two detectors to continuously and simultaneously measure both tissue oxygen saturation (rSO2) and arterial blood oxygenation (SpO2). Root allows either one or two O3 MOC-9 modules to be connected, enabling monitoring with as few as one and as many as four sensors.
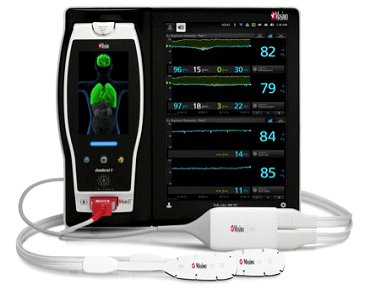
Masimo O3™ regional oximetry MOC-9 module for the Root™ patient monitoring and connectivity platform.
Regional oximetry, also referred to as tissue oximetry and cerebral oximetry, enables the continuous assessment of the oxygenation of the tissue beneath the sensor. O3 helps clinicians detect regional hypoxemia that pulse oximetry alone can miss. In addition, the onboard pulse oximetry capability in O3 sensors can automate the differential analysis of regional to central oxygen saturation. O3 monitoring is as simple as applying O3 regional oximetry sensors to the forehead and connecting the O3 MOC-9 module to any Root through one of its three MOC-9 ports. Root offers multiple unprecedented, high-impact innovations including:
- Radical-7® with Masimo's breakthrough rainbow® and SET® measurements
- Instantly interpretable, high visibility, intuitive-navigation touchscreen display
- MOC-9 flexible measurement expansion with SedLine® EEG brain function monitoring and Phasein™ capnography, in addition to O3 regional oximetry and future measurements
- Iris™ for built-in connectivity gateway for standalone devices such as IV pumps, ventilators, hospital beds, and other patient monitors
- MyView™ for automatic display of parameters, waveforms, and viewing configuration based on the clinician's preference and presence
Masimo O3 sensors enable simultaneous measurement of regional oxygen saturation (rSO2) and arterial oxygen saturation (SpO2).
In an abstract presented at the Society for Technology in Anesthesia 2014 Annual Meeting in Orlando, Fla., Dr. Daniel Redford from the University of Arizona evaluated cerebral oxygen saturation on 23 subjects and 202 paired measurements of rSO2 from O3 regional oximetry and reference arterial and venous blood samples (SavO2).1 Reference blood samples were taken from both an arterial cannula placed in the radial artery and a catheter placed in the internal jugular bulb vein, obtained at baseline and after a series of increasingly hypoxic states. O3 regional oximetry had an absolute accuracy of 4.0% and trend accuracy of 2.1%.
"Masimo O3 regional oximetry will have the unique ability to measure both rSO2 and Masimo SET® SpO2 pulse oximetry simultaneously from the same forehead sensor," said Dr. Michael Ramsay, M.D., Chief of the Department of Anesthesiology and Pain Management at Baylor University Medical Center in Dallas. "This may provide the anesthesiologist or perfusionist for the first time with a differential analysis of regional to central oxygen saturation monitoring that could help the clinician in maintaining brain oxygenation and safe cerebral perfusion during cardiac procedures."
O3 regional oximetry is currently intended for use in subjects larger than 40 kg (88 lbs) and has not yet received FDA 510(k) clearance.
Joe Kiani, CEO and Founder of Masimo, said, "O3 regional oximetry delivers again on Masimo's mission to improve patient outcomes and reduce cost of care by taking noninvasive monitoring to new sites and applications. We look forward to partnering with key hospitals around the world to demonstrate O3's technical and clinical advantages."
1 Redford D, Paidy S, Kashif F, STA 2014; 46 (abstract);
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to help clinicians detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow ® Pulse CO-Oximetry™ technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures; total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), PVI®, and perfusion index (PI), in addition to measure-through motion SpO2, and pulse rate. In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic Monitoring™, the first-ever commercially available noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo SET® and Masimo rainbow® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results, risks related to our belief that Masimo O3 Regional Oximetry provides clinically acceptable absolute and trend accuracy, risks related to our belief that O3 Regional Oximetry simultaneously supports critical regional cerebral oxygen saturation and blood oxygenation, and that it helps ensure safe, adequate cerebral perfusion of the patient's brain, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Mike Drummond
Masimo Corporation
Phone: (949) 297-7434
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57, Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SEDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.


Masimo Brings High Technology Medical Equipment to India
Expands Presence with Advanced Noninvasive Patient Monitoring
Bangalore, India & Irvine, California – January 7, 2014 – Masimo (NASDAQ: MASI), the inventor of breakthrough noninvasive patient monitoring technologies, today announced the opening of offices across India to meet the health demands of the burgeoning Indian market.
Masimo, a successful, publicly traded medical technology company employing more than 3,000 people worldwide with product revenues that have steadily increased in the last five years, has technology license and OEM agreements with leading patient monitoring manufacturers spanning the globe, including Atom, Datascope, GE Medical, Medtronic, Philips, Spacelabs, and Zoll.
"We have found that India is a an important market for several reasons – the most important is that the penetration of high technology devices is very low, a factor that could lead to thousands of avoidable instances of death and illness. As a leader in noninvasive patient monitoring, including measure through motion and low perfusion pulse oximetry, Masimo's technologies will be able to make a significant impact to improve patient safety and outcomes," said Jon Coleman, President, Masimo Worldwide Sales, Professional Services and Medical Affairs.
Masimo is working with India's top medical and nursing practitioners to raise greater awareness of the clinical and cost challenges that Masimo's innovative technologies can solve, including:
- Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry. Compared to other pulse oximeters during patient motion and low perfusion, Masimo SET® provides measurements when other pulse oximeters cannot, dramatically reduces false alarms (specificity), and accurately detects true alarms (sensitivity)1,2 that can indicate a deteriorating patient. Most importantly, Masimo SET® pulse oximetry has been shown to improve patient outcomes by helping clinicians reduce retinopathy of prematurity (ROP)3 in neonates, screen newborns for critical congenital heart disease (CCHD),4,5 reduce ventilator weaning time and arterial blood gas measurements in the ICU,6 and save lives and costs while reducing rapid response activations and intensive care unit transfers on the general floor.2
- rainbow® Pulse CO-Oximetry™, which allows clinicians to noninvasively monitor multiple blood constituents, such as
- Total hemoglobin (SpHb®), when used in conjunction with laboratory testing, SpHb monitoring can help clinicians reduce unnecessary blood transfusions in both low and high blood-loss surgery situations, and also helps initiate more timely transfusions when they are truly indicated7,8;
- Carboxyhemoglobin (SpCO®), shown to be useful as a first-line assessment tool in helping clinicians rapidly detect carbon monoxide (CO) poisoning9;
- Methemoglobin (SpMet®), shown to help clinicians assess for methemoglobinemia10 – a dangerous reaction to some hospital-administered drugs that reduces the delivery of oxygen to the tissues. Other breakthrough technologies include:
- RAM™ acoustic respiration rate (RRa™), which noninvasively and continuously measures respiration rate using an innovative adhesive sensor with an integrated acoustic transducer that is easily and comfortably applied to the patient's neck, and shown to be as effective as capnography (nasal cannula) and better tolerated by patients11;
- Root™, a powerful new patient monitoring and connectivity platform that integrates breakthrough rainbow® and SET® measurements with multiple additional parameters—including SedLine® brain function monitoring and Phasein™ capnography and gas monitoring.* Root includes a dock for the Radical-7® handheld Pulse CO-Oximeter and acoustic respiration rate monitor, an instantly interpretable display, and multiple networking/connectivity options. Root integrates multiple streams of data and simplifies patient care workflows, empowering caregivers to help make quicker patient assessments, earlier interventions, and better clinical decisions; and
- Masimo Patient SafetyNet™, a remote monitoring and clinician notification system clinically shown to keep patients safer by enabling remote continuous monitoring of the patient's physiology, including oxygenation and pulse rate, which has led to saved lives, reductions in rapid response activations and transfers to intensive care units.12
Local response from the clinical community has been overwhelming.
"I've been using Masimo technology for the last three years or so and am very happy to hear about Masimo's commitment to the region," said Dr. Anil Karlekar, executive director and head of anesthesiology and critical care at Fortis Escorts Heart Institute in New Delhi. "I look forward to closer collaboration with the Masimo India team."
"I have found that Masimo's technology is best in picking up SpO2 in compromised babies, particularly when very sick," said Dr. Ashish Mehta of Arpan Nursing Home, in Ahemdabad, where he and his team focus on pre-term newborns and care for more than 600 babies a year. "I rely on this technology in my clinic and am very happy to learn that Masimo is now in India and better poised to serve its local clients."
"I'm glad to learn that Masimo has arrived in India through its own sales and clinical team," added Dr. Kartik Nagesh, head of neonatology at Manipal Hospital in Bangalore. "We have been using Masimo technology in our NICU for some years and now with a local presence we are more confident about our ability to improve quality of care to our neonatal patients."
1 Shah N, Ragaswamy HB, Govindugari K, Estanol L "Performance of Three New-Generation Pulse Oximeters during Motion and Low Perfusion in Volunteers".J Clin Anesth. 2012 Aug;24(5):385-91.
2 Taenzer, Andreas H.; Pyke, Joshua B.; McGrath, Susan P.; Blike, George T. "Impact of Pulse Oximetry Surveillance on Rescue Events and Intensive Care Unit Transfers: A Before-and-After Concurrence Study." Anesthesiology, February 2010, Vol. 112, Issue 2. For further reading visit: https://pubs.asahq.org/anesthesiology/article/132/3/602/108898/Impact-of-Pulse-Oximetry-Surveillance-on-Rescue.
3 Castillo A, et al. Acta Paediatr. 2011 Feb.;100(2):188-92.
4 de-Wahl Granelli A., et al. BMJ. 2009 Jan 8;338.
5 Ewer A, et al. Health Technol Assess. 2012;16(2):1-184.
6 Durbin, et al. Critical Care Medicine. 2002 Aug.;30(8): 1735 to 1740.
7 Ehrenfeld JM et al. ASA. 2010. LB05. (abstract)
8 Awada WNFM et al. Anesth Analg 2013; (117 suppl): 50 (abstract).
9 Sebbane M, Claret PG, Mercier G, Lefebvre S, Thery R, Dumont R, et al. "Emergency Department Management of Suspected Carbon Monoxide Poisoning: Role of Pulse Co-Oximetry." Respir Care. Published online ahead of print Mar 19, 2013.
10 Annabi E.H., Barker S,J. Anesth Analg. 2009;108:898–9.
11 Patino M., Redford D.T., Quigley T.W., Mahmoud M., Kurth C.D., Szmuk P. Accuracy of acoustic respiration rate monitoring in pediatric patients. Pediatric Anesthesia. 2013 Sep 3.
12 Taenzer A, Blike G, McGrath S, Pyke J, Herrick M, Renaud C, Morgan J. "Postoperative Monitoring – The Dartmouth Experience." Anesthesia Patient Safety Foundation Newsletter Spring-Summer 2012. https://www.apsf.org/article/postoperative-monitoring-the-dartmouth-experience/
*Root is CE Marked
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). Additional information about Masimo and its products may be found at www.masimo.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our belief that Masimo SET provides real-time results for all patients to help clinicians to more rapidly assess, diagnose, and treat every patient; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including: total hemoglobin (SpHb), SpCO, SpMet, and RRa contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Bharat Monteiro
Masimo Corporation
Phone: 1800-425-MASIMO (627466)
Email: [email protected]
Archana Muthappa (India)
Adfactors PR Pvt Ltd
Phone: 91 44 42655800
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI, rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5, Pulse CO-Oximetry, Pulse CO-Oximeter, Adaptive Threshold Alarm, and SeDLine are trademarks or registered trademarks of Masimo Corporation. The use of the trademarks Patient SafetyNet and PSN are under license from University HealthSystem Consortium.

Masimo to Present at 32nd Annual J.P. Morgan Healthcare Conference
- Irvine, California, January 3, 2014 –Masimo (NASDAQ: MASI) today announced that its management is scheduled to present at the 32nd Annual J.P. Morgan Healthcare Conference at the Westin St. Francis Hotel in San Francisco on Wednesday, January 15, 2014, at 2:00 p.m. Pacific Time. A live audiocast of the presentation will be available on the Masimo website at www.masimo.com. A replay of the audiocast will be available following the live presentation.
About Masimo
Masimo (NASDAQ: MASI) is the global leader in innovative noninvasive monitoring technologies that significantly improve patient care—helping solve "unsolvable" problems. In 1995, the company debuted Measure-Through Motion and Low Perfusion pulse oximetry, known as Masimo SET®, which virtually eliminated false alarms and increased pulse oximetry's ability to detect life-threatening events. More than 100 independent and objective studies have shown that Masimo SET® outperforms other pulse oximetry technologies, even under the most challenging clinical conditions, including patient motion and low peripheral perfusion. In 2005, Masimo introduced rainbow SET® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously required invasive procedures, including total hemoglobin (SpHb®), oxygen content (SpOCTM), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and Pleth Variability Index (PVI®), in addition to SpO2, pulse rate, and perfusion index (PI). In 2008, Masimo introduced Patient SafetyNet™, a remote monitoring and wireless clinician notification system designed to help hospitals avoid preventable deaths and injuries associated with failure to rescue events. In 2009, Masimo introduced rainbow® Acoustic MonitoringTM, the first-ever noninvasive and continuous monitoring of acoustic respiration rate (RRa™). Masimo's rainbow® SET® technology platform offers a breakthrough in patient safety by helping clinicians detect life-threatening conditions and helping guide treatment options. In 2010, Masimo acquired SEDLine®, a pioneer in the development of innovative brain function monitoring technology and devices. And in 2012, Masimo acquired the assets of Spire Semiconductor, LLC, a maker of advanced light emitting diode (LED) and other advanced component-level technologies; and PHASEIN AB, a developer and manufacturer of ultra-compact mainstream and sidestream capnography, multigas analyzers, and handheld capnometry solutions. Masimo SET® and Masimo rainbow® SET® technologies also can be found in over 100 multiparameter patient monitors from over 50 medical device manufacturers around the world. Founded in 1989, Masimo has the mission of "Improving Patient Outcome and Reducing Cost of Care … by Taking Noninvasive Monitoring to New Sites and Applications®." Additional information about Masimo and its products may be found at www.masimo.com.
# # #
Investor Contact:
Eli Kammerman
(949) 297-7077
[email protected]
Media Contact:
Mike Drummond
(949) 297-7434
[email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care… by Taking Noninvasive Monitoring to New Sites and Applications, Rainbow, SpHb, SpOC, SpCO, SpMet, PVI, Rainbow Acoustic Monitoring, RRa, Radical-7, Rad-87, Rad-57,Rad-8, Rad-5,Pulse CO-Oximetry, Pulse CO-Oximeter, and SEDLine are trademarks or registered trademarks of Masimo Corporation.