News & Media
2022
Home / News & Media / 2022
2022
NEWS & MEDIA:

Masimo Takes Major Step in Connecting the Hospital to the Home
Breakthrough Ecosystem Expansion to Seamlessly Integrate Continuous Health Data from Masimo Devices with Four Million HEOS-Enabled Devices Launches
Irvine, California - December 13, 2022 - Masimo (NASDAQ: MASI) today announced the launch of the expansion of the HEOS platform to provide a robust and always-on connection to the Masimo Health secure cloud. This feature adds the ability to aggregate, record, and display health data from wearable Masimo continuous and spot-check health and wellness devices. Leveraging the HEOS ecosystem, the Masimo Health secure cloud solution will seamlessly be made available to the users of over 4 million HEOS-enabled premium devices from Denon, Marantz, and Definitive Technology.
Allowing users unprecedented access to secure, continuous health data from the comfort of home, and facilitating their ability to share data with clinicians and loved ones, Masimo’s latest innovation will initially be made available via software upgrade in over 20,000 HEOS Denon Home devices and Masimo Home Health Hubs in the U.S. An additional four million devices around the world are planned for activation in 2023.
Masimo is bringing together synergies in health, cloud-based IoT, and entertainment technologies to create a rich ecosystem designed to revolutionize how we connect and interact with health technologies, including the availability of Masimo’s latest wearable monitoring devices. Using this powerful software platform, the Masimo W1™ advanced health tracking watch, Masimo SafetyNet® patient management solution with Radius PPG® and Radius T°® sensors, and many more devices, will not only wirelessly relay data to the HEOS and Masimo Health cloud for storage, analysis, reporting, and transfer, but make this data available via in-home displays and apps, alongside customizable notifications and reminders.
To ensure that health data remains safe, secure, and reliably available, Masimo’s HIPAA-compliant ecosystem provides safeguards such as redundancy in communication with the cloud. As users move throughout the home, and even beyond it, key health data, including oxygen saturation, pulse rate, respiration rate, hydration, and temperature, are available continuously, allowing users to track their health and wellness. The platform will also support the hosting of telehealth sessions from home, with data transfer to clinicians streamlined to assist them in providing the most comprehensive remote care possible.
Joe Kiani, Founder and CEO of Masimo, said, “Today represents a major milestone in the integration of our iconic, premium audio brands and our revolutionary healthcare products designed to save and improve lives. Leveraging Masimo’s clinically proven monitoring technologies, alongside the state-of-the-art engineering and reach of the HEOS technology platform, Masimo plans to transform existing healthcare, safety, and entertainment paradigms to improve life in the hospital and in the home.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and the Masimo W1™ watch. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo HEOS and Masimo Health Hubs. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo HEOS and Masimo Health Hubs, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks that Masimo fails to make its latest innovation available in HEOS Denon Home devices in the U.S. and Masimo Home Health Hubs as indicated in this press release, or to activate devices in 2023 as planned; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces Limited Market Release of Sepsis Index
Breakthrough Early Warning Solution, for Integration with Masimo Patient SafetyNet™, Is Designed to Help Clinicians Catch Deterioration in Patient Condition
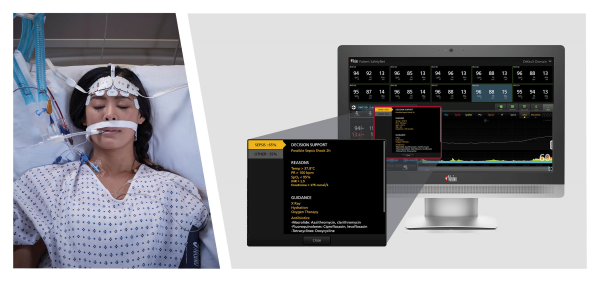
Neuchate, Switzerland - December 12, 2022 - Masimo (NASDAQ: MASI) today announced the limited market release of Sepsis Index (Si™), an early warning indicator designed to help clinicians identify possible sepsis in patients remotely monitored with Masimo Patient SafetyNet™. Patient SafetyNet, a powerful remote patient monitoring and surveillance system in use around the world, aggregates real-time physiological data from Masimo and third-party bedside monitoring equipment for display at central stations, allowing clinicians to monitor patient condition from afar. By continuously analyzing a variety of vital signs data and other clinical measurements together with EMR data captured by Patient SafetyNet, Si works by quantifying patient status in an easily interpretable sepsis risk score.
Sepsis Index, using the aggregated data from Patient SafetyNet, is designed to provide early warning of possible sepsis or other causes of deterioration in a patient’s condition, and to help track patient status following sepsis diagnosis and monitor the effectiveness of treatment and interventions. In addition, Si is designed to display on-screen decision support and recommended actions that each institution can update according to its sepsis policy. The Si algorithm is customizable and draws upon Masimo’s expertise in developing a variety of advanced early warning risk-scoring solutions, such as Halo and Halo ION®, since 2009.
Sepsis, a life-threatening condition in which organs malfunction because of widespread infection, is common among hospital patients and is a common cause of death.1 The CDC estimates that every year, about 1.7 million adults in the U.S. develop sepsis, of whom at least 350,000 die during their hospitalization or in hospice. One in every three patients who dies in a U.S. hospital had sepsis during their hospitalization.2 Sepsis has also been described as the most expensive condition to treat in the entire U.S. healthcare system, with an estimated $24 billion in annual costs.3 Early detection of sepsis has been associated with decreased mortality.4 Masimo Si is designed to help clinicians detect physiologic deterioration associated with the onset of sepsis and track the progress of the patient’s condition during its duration.
Aldo Carmona, MD, Senior Vice President of Clinical Innovation and Chairman of the Department of Anesthesia and Critical Care at St. Luke’s University Health Network, said, “Although there have been great advancements both clinically and technically, assessment and early identification of sepsis remain an elusive challenge. Masimo’s new indicator, Si, provides a continuous and real-time assessment of sepsis risk for a given patient based on the clinical measurements available from monitors and other EMR data (labs, medications, etc.). This could be a great addition in a hospital’s fight against the leading cause of in-patient mortality. By alerting the clinical staff to the risk of potential sepsis, it would allow for more timely intervention with life-saving resuscitation and medication. Likewise, tracking the patient state will help assess the effects of treatment and interventions so that we can improve appropriate allocation of hospital resources to patients and potentially identify patients who need escalation of treatment earlier.”
Joe Kiani, Founder and CEO of Masimo, “Masimo has always sought to solve those ‘unsolvable’ problems that need resolution. Detection of sepsis has long been one of the unsolved clinical problems, and one which we have spent considerable time and effort to remedy. With Sepsis Index, we hope to help doctors and nurses everywhere identify sepsis before it’s too late to treat it.”
Sepsis Index (Si) is not FDA cleared.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,6 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-11 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,12 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.13 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and the Masimo W1™ watch. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Singer M et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016.
- https://www.cdc.gov/patientsafety/features/get-ahead-of-sepsis.html#:~:text=About%201.7%20million%20adults%20in,had%20sepsis%20during%20that%20hospitalization
- https://www.sepsis.org/news/new-u-s-government-report-reveals-annual-cost-of-hospital-treatment-of-sepsis-has-grown-by-3-4-billion/
- Kim HI, Park S. Sepsis: Early Recognition and Optimized Treatment. Tuberc Respir Dis (Seoul). 2019 Jan;82(1):6-14. doi: 10.4046/trd.2018.0041. Epub 2018 Sep 28. PMID: 30302954; PMCID: PMC6304323.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Si™ and Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Si and Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces Full Market Release of Hydration Index for the Masimo W1™ Advanced Health Tracking Watch
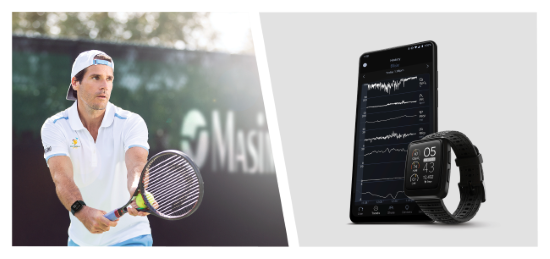
Irvine, California - December 7, 2022 - Masimo (NASDAQ: MASI) today announced the full market release of Hydration Index (Hi™) for the Masimo W1™ watch. The Masimo W1, an advanced health tracking wearable, is the first watch to offer accurate, continuous pulse oximetry measurements and insightful health data, from the leader in hospital pulse oximetry. Hydration Index, first announced earlier this year in a limited market release, provides an index that tracks an individual’s hydration levels and integrates seamlessly with other continuous Masimo W1 health data, such as oxygen saturation (SpO2), pulse rate, respiration, and more.
Hydration level has been one of the most sought out parameters by athletes, vocalists, and others seeking to optimize their performance. Since creating PVi® – which allows clinicians to assess fluid responsiveness of mechanically ventilated patients – nearly 15 years ago, Masimo has been working to invent a way to help people gain insight into hydration. Proper hydration is widely recognized as an important aspect of health and performance, and lack of proper hydration affects many physiological parameters, as the body works to restore homeostasis. Masimo W1 is designed to help you identify your hydration baseline, helping you understand your hydration level, which not only affects athletic performance, but can also support healthier lifestyle decisions. Whether you’re an elite athlete, a vocalist, a health-focused individual, or just keen to gain more insight into your body’s physiological status, Masimo W1 with Hydration Index represents a breakthrough solution to better understand and manage hydration health.
Tommy Haas, Olympic silver medalist and professional tennis player, said, “I have wanted to know the level of my hydration since I began competing, but always had to guess about it. Masimo W1 with Hydration Index finally makes this possible.”
Nick Mayhugh, World Record holder and three-time Paralympic champion, added, “As a professional athlete who trains six days a week and ten hours a day, I need to know my hydration levels at all times. Masimo’s Hydration Index technology will provide athletes of all levels the opportunity to maximize their training and recovery programs, leading to optimal performance.”
Known for its exceptional accuracy and reliability during challenging conditions, such as motion and low perfusion, Masimo, with the Masimo W1, brings its expertise in signal processing, photonics, and bio-sensing to consumers looking to take control of their personal health, make better health decisions, and monitor their overall physiological status. Masimo W1 pairs via secure Bluetooth® to the Masimo Health™ smartphone app to unlock meaningful, actionable insights. The integrated Personal SafetyNet™ subscription service gives users access to sophisticated reporting tools to help them review their physiological status over time and facilitates sharing data with family members, fitness trainers, wellness coaches, and where allowed, healthcare providers.
Benefiting from Masimo’s expertise in hospital connectivity and hospital automation, a medical version of Masimo W1 will be available outside the US for use in telehealth and telemonitoring applications via Masimo SafetyNet® and Personal SafetyNet™ for healthcare providers and payers, as well as individual use. Masimo W1 is a convenient, reliable remote monitoring and telehealth solution enabling hospitals and clinicians to proactively keep track of their patients’ physiological status from afar, even as patients go about everyday tasks at home. A natural complement to the Masimo SafetyNet remote patient monitoring platform, Masimo W1 enables wireless transmission of patient data to the Masimo SafetyNet app and Masimo’s secure data cloud, where it can be reviewed in near-real time by remote monitoring teams in centralized locations for signs of physiological decline or sudden changes, such as falls or spikes in heart rate.
Dr. Amin, Professor, Endowed Chair of Medicine and Executive Director of Hospital Medicine at the University of California, Irvine, said, “Evaluating intravascular volume status is a common problem in clinical care, particularly for post-op patients and those with complicated chronic conditions such as congestive heart failure (CHF). These patients often seek care in the emergency department (ED) for monitoring and treatment. Masimo W1 helps individuals understand their hydration level by tracking their Hydration index (Hi), and provides a common additional monitoring endpoint beyond daily weights for evaluation of hydration. Having continuous, noninvasive monitoring of Hi available in a wearable device means people are now able to access important, real-time information at home. This has the potential to be a game changer for all involved in the management of chronic conditions like CHF, and also for those managing post-op patients.”
Joe Kiani, Founder and CEO of Masimo, said, “Masimo W1 represents numerous firsts: the first wrist-worn wearable device to offer consumers accurate, continuous pulse oximetry. The first – but not the last – such device from Masimo. And, with Hydration Index, the first watch to give users actionable, insightful hydration data, helping everyone to make healthier decisions so that they can live their best lives.”
Masimo W1 and Hi have not been cleared by the FDA and are not available for use in medical applications in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and the Masimo W1™ watch. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- America's Best Hospitals: the 2022-2023 Honor Roll and Overview. health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®, Masimo PVi®, and Masimo Radical-7® Pulse CO-Oximeters®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, Masimo PVi, and Masimo Radical-7 Pulse CO-Oximeters, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

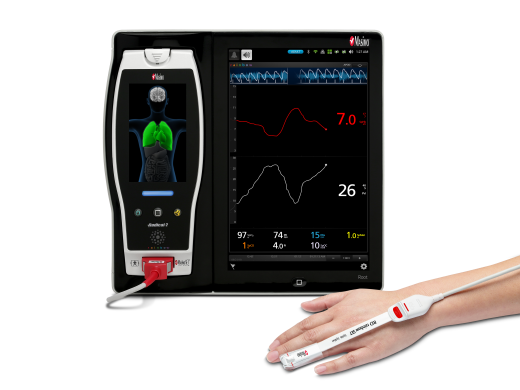
New Prospective Study Finds That Masimo SpHb® Noninvasive and Continuous Hemoglobin Monitoring Can Help Provide Effective Blood Management in Patients Undergoing Major Surgery
Neuchatel, Switzerland - November 30, 2022 - Masimo (NASDAQ: MASI) today announced the findings of a prospective, double-blinded, randomized, controlled trial published in the Journal of the College of Physicians and Surgeons Pakistan. In the study, Dr. Sukriye Akdag and colleagues at Marmara University in Istanbul, Turkey assessed the impact of noninvasive and continuous hemoglobin monitoring with Masimo SpHb® on blood transfusion management for adult patients undergoing elective major surgery with anticipated blood loss of 20% or more. The researchers found that the postoperative red blood cell (RBC) transfusion rate was lower, and the hemoglobin level of ICU patients higher, when monitored with SpHb in the operating room. They concluded, “SpHb can provide effective patient blood management in cases of major surgery.”1
Noting that delayed or unnecessary blood transfusions have been associated with increased mortality and morbidity, the researchers sought to evaluate the impact of continuous hemoglobin monitoring with Masimo SpHb, which offers continuous trending of hemoglobin levels, provided noninvasively and in real time. The researchers enrolled 120 patients aged 18-85 (ASA score I-III) scheduled for major surgery, divided randomly into an SpHb group (n=60) and a control group (n=60). There were no significant differences in the demographics or mean arterial pressure, heart rate, or arterial blood gas analysis between the groups. In the control group, patients’ hemoglobin was measured using conventional, intermittent blood sampling, analyzed with an ABL800Flex Radiometer, at the beginning, the second hour, the fourth hour, and the end of the operation. In the SpHb group, in addition to conventional blood sampling, patients’ SpHb values, pleth variability index (Masimo PVi®) and perfusion index (Pi) were noninvasively and continuously monitored with Masimo Radical-7® Pulse CO-Oximeters®; in the case of a sudden decrease in hemoglobin value, an additional blood gas analysis was performed. In both groups, transfusion was carried out when hemoglobin levels fell below 9 g/dL (per standard European Society of Anaesthesia recommendations) by blood gas analysis.
The researchers found that, comparing overall postoperative measurements, there were no significant differences between the groups in hemoglobin, platelet, or creatinine levels; nor in the amount of fresh frozen plasma, or platelet suspension transfused intraoperatively and postoperatively; nor in the amount of RBC units transfused intraoperatively. However, the postoperative RBC transfusion rate in the SpHb group was significantly lower (SpHb group: median 0 international units (IUs); control group: median 2 IUs; p=0.020).* Postoperative hemoglobin levels in SpHb group patients in the ICU were also statistically significantly higher (SpHb group: 8.41 g/dL ± 1.08 g/dL; control group: 7.75 g/dL ± 1.19 g/dL; p=0.033).
The investigators concluded, “SpHb measurement in major surgical cases can accompany conventional Hb measurement methods, allowing effective patient blood management practice. … Since this may decrease mortality and morbidity by reducing postoperative blood transfusion, the use of such an advanced monitoring method in major surgeries may increase patient safety.”
This study adds additional evidence to the growing literature on the value of continuous hemoglobin monitoring with SpHb. SpHb, as part of patient blood management programs, has been found to improve outcomes in both high- and low-blood loss surgeries, such as reducing the percentage of patients receiving allogeneic transfusions,2,3 reducing the units of red blood cells transfused per patient,4-6 reducing the time to transfusion,7 reducing costs,8 and even reducing mortality 30 and 90 days after surgery by 33% and 29%, respectively (when combined with a goal-directed fluid therapy algorithm using Masimo PVi).9 This evidence of SpHb’s impact on outcomes spans the globe, now representing 7 countries on 4 different continents.1-9 Today, Masimo SpHb technology supports clinicians and patient care in more than 75 countries.
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
*The transfusion rate data were provided by the authors after study publication and are included here with their permission.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.10 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,11 improve CCHD screening in newborns,12 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.13-16 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,17 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.19 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and the Masimo W1™ watch. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Akdag S, Zengin SU, Cakmak G, Umuroglu T, Aykac ZZ, Saracoglu A. Targeted Bleeding Management Guided by Non-Invasive Haemoglobin Measurement in Surgical Patients. J Coll Physicians Surg Pak 2022; 32(10):1242-1248.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Nakamori E et al. Geriatr Orthop Surg Rehabil. 2021 Nov 19;12:21514593211060575.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Merolle L, Marraccini C, Di Bartolomeo E, Montella M, Pertinhez T, Baricchi R, Bonini A. Postoperative patient blood management: transfusion appropriateness in cancer patients. Blood Transfus 2020; 18: 359-65 DOI 10.2450/2020.0048-20.
- Saracoglu A, Abdullayez R, Sakar M, Sacak B, Incekoy F, Aykac Z. Continuous hemoglobin measurement during frontal advancement operations can improve patient outcomes. J Clin Mon Comp. 7 Mar 2022. www.doi.org/10.1007/s10877-022-00813-5.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. www.doi.org/10.1007/s10877-019-00367-z.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009; Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®, Masimo PVi®, and Masimo Radical-7® Pulse CO-Oximeters®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, Masimo PVi, and Masimo Radical-7 Pulse CO-Oximeters, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Peer-Reviewed Study Finds That Masimo SET® Pulse Oximetry Has No Clinically Significant Difference in Accuracy or Bias Between Black and White People
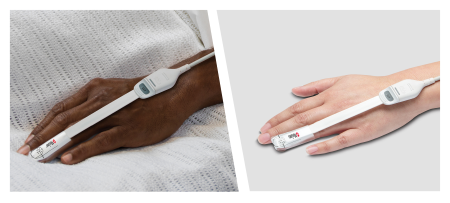
Irvine, California - November 21, 2022 - Masimo (NASDAQ: MASI) today announced the publication of a peer-reviewed study regarding Masimo SET® pulse oximetry performance in varying skin pigmentation in the Journal of Clinical Monitoring and Computing. The retrospective trial, "Racial effects on Masimo pulse oximetry: a laboratory study," by Drs. Steven J. Barker and Wilson C. Wilson, found that there was no clinically significant difference in the accuracy or bias between Black and White subjects studied with Masimo SET® pulse oximetry and Masimo RD SET® sensors.1
For this newly published study Dr. Barker (Chief Science Officer, Masimo) and Dr. Wilson (Chief Medical Officer, Masimo) performed a retrospective analysis of Masimo laboratory data obtained from self-identified Black and White volunteer subjects, to evaluate differences in Masimo pulse oximeter accuracy and bias on the basis of skin tone. The investigators reviewed data collected between October 2015 and July 2021, which included 7,183 paired samples (3,201 Black and 3,982 White) collected from 75 subjects (39 Black and 36 White), who were screened with the same criteria to remove potential bias based on health conditions. All subjects were exposed to the same hypoxia protocol, which varied the arterial saturation of hemoglobin (SaO2) between 70% and 100%. Noninvasive oxygen saturation (SpO2) values were obtained from Masimo SET® pulse oximeters with RD SET sensors and time-matched with simultaneously taken arterial blood gas (ABG) samples analyzed using an ABL-835 blood gas analyzer.
The data were analyzed to determine the bias (mean difference in paired SpO2 and SaO2 samples), precision (standard deviation of the difference), and accuracy (root mean squared error, ARMS*) for both groups. A negative bias of 0.20% was found for Black subjects, compared to 0.05% for White subjects. This difference of 0.15% (p < 0.001) is not clinically significant and the values are numerically indistinguishable because the SpO2 display resolution is 1% on commercially available pulse oximeters (both from Masimo and other manufacturers). The investigators also found a precision of 1.40% for Black subjects and 1.35% for White subjects. Accuracy (ARMS) was 1.42% for Black subjects and 1.35% for White subjects. These results are consistent with the accuracy specifications of RD SET sensors (1.5% accuracy ARMS), which are twice as good as the current FDA clearance thresholds for medical-grade pulse oximeters (3.0% accuracy ARMS).
In discussing their findings, Drs. Barker and Wilson describe how Masimo SET® accounts for skin pigmentation when measuring SpO2. "The absence of racial bias, and highly accurate overall performance exhibited by Masimo SET® pulse oximetry can be logically explained by Masimo's engineering design and testing paradigm. ... Conventional pulse oximetry uses the standard red over infrared algorithm to provide SpO2, while Masimo SET® uses that conventional algorithm along with four additional signal processing engines that all run in parallel. These signal processing systems allow the distinction between arterial and venous signal during motion and low perfusion by identifying and isolating the non-arterial and venous noise SpO2 from the true arterial SpO2 components in the signal. These multiple signal processing engines work together to overcome limitations of each independent method. This advanced technique allows for a more accurate picture of the pulsatile (arterial) signal and significantly reduces the impact of static absorbers such as skin pigment, bone density, and tissue thickness (e.g., finger, toe, or earlobe). Finally, the Masimo SET® SpO2 algorithm is calibrated and then validated using nearly equal numbers of dark and light-skinned subjects."
Drs. Barker and Wilson summarized, "In conclusion, this retrospective study of healthy human volunteers monitored with Masimo RD SET pulse oximeter sensors showed an absence of clinically significant differences in accuracy between Black and White subjects." The authors suggested that additional prospective clinical studies should be conducted to validate their results in critically ill patients utilizing Masimo SET® pulse oximeters and those from other manufacturers.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within +/- ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. In addition, Masimo Consumer Audio is home to eight legendary audio brands, including Bowers & Wilkins, Denon, Marantz, and Polk Audio. Our mission is to improve life, improve patient outcomes, and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.10 In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7®, Radius PPG®, and Radius VSM™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital and home automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Its growing portfolio of health and wellness solutions includes Radius T°® and the Masimo W1™ watch. Additional information about Masimo and its products may be found at www.masimo.com. Published clinical studies on Masimo products can be found at www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Barker SJ, Wilson WC. Racial effects on Masimo pulse oximetry: a laboratory study. J Clin Monit Comput. 12 Nov 2022. doi.org/10.1007/s10877-022-00927-w.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® and RD SET®, and Masimo’s plan to conduct additional studies on the accuracy of Masimo SET® and RD SET on Black people and White people ("Additional Studies"). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SET® and RD SET, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Sets the Record Straight on Bylaw Amendments
Responds to Politan Capital Management’s Complaint
IRVINE, Calif.--(BUSINESS WIRE)-- Masimo today issued the following statement in response to the complaint filed by Politan Capital Management (“Politan”) in the Delaware Court of Chancery (the “Lawsuit”):
We believe this lawsuit is being initiated after the Masimo Board refused to accede to Politan’s strongarm demands for Board representation, including its demand for a board seat for Politan’s founder, Quentin Koffey, who has never served on a corporate board and has no relevant experience in Masimo’s industry. Politan accumulated a nearly 9% stake in Masimo without any prior dialogue with the Company, and its lawsuit is an effort to disguise its self-serving agenda. Masimo’s Board continues to be highly focused on serving the best interests of all shareholders.
The bylaw amendments were adopted by the Masimo Board after thoughtful consideration. They are designed to improve transparency to ensure that stockholders receive information relevant to an informed vote. We reject the premise that an activist hedge fund attacking a public company should be allowed to hide its web of financial entanglements and significant financial backers.
Quinn Emanuel Urquhart & Sullivan LLP, Paul Hastings LLP and Abrams & Bayliss are acting as legal counsel to the Company.
About Masimo
Masimo (Nasdaq: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates, improve CCHD screening in newborns, and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs. Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world, and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll. Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at professional.masimo.com. Published clinical studies on Masimo products can be found at professional.masimo.com/evidence/featured-studies/feature/.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the lawsuit filed by Politan and Masimo’s bylaw amendments. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to, risks related to litigation, as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221021005429/en/
Investor Contact:
Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact:
Jamie Moser / Meaghan Repko / Fouad Boutros / Tali Epstein
Joele Frank, Wilkinson Brimmer Katcher
Phone: (212) 355-4449

Masimo Appoints New Leader for Consumer Division
Blair Tripodi Named Chief Operating Officer for Masimo Consumer
Irvine, California - September 19, 2022 - Masimo Masimo (NASDAQ: MASI) today announced the appointment of Blair Tripodi as Chief Operating Officer (COO) for the Masimo Consumer division, effective immediately. As leader of the Masimo Consumer unit, Tripodi will report to Masimo's Founder and CEO, Joe Kiani, and will be responsible for the division’s sales, product, marketing, and commercial operations teams. Tripodi's most recent role was Chief Commercial Officer at Sound United.
"As we embark on this new era of Masimo, I couldn't be more excited for the future of our company," said Joe Kiani. "Blair's leadership, history, and extensive background in marketing, sales, and consumer products will position Masimo's consumer business, including the coming consumer health products, for optimal growth. His years of experience running successful campaigns in the consumer space, including 10 years at Sound United, will not only further our whole home audio solutions but will further our plans to improve 21st-century healthcare by taking it directly into your home."
Blair Tripodi joined Sound United in 2013. Prior to joining the global home and audio company, he was the Managing Director of Under Armour's European, Middle Eastern, and African business. In addition, he has held a variety of marketing roles with Nike and the U.S. Olympic and Paralympic Committee.
"It's an honor to step up and lead our consumer team into the next phase of growth with new innovative audio products as well as life-improving consumer healthcare products," Tripodi commented. "We can do this because of our dedicated team, loyal customers, and global partner network. There are exciting times ahead as we set out to improve lives."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Medical Pioneer Masimo Announces the Full Market Consumer Release of the Masimo W1™, the First Watch to Offer Accurate, Continuous Health Data
Also Available, in Limited Market Release, Is Masimo W1 with Hydration Index, Designed to Provide Insight Into an Individual's Hydration
Irvine, California - August 31, 2022 - Masimo Masimo (NASDAQ: MASI) today announced the full market release of the Masimo W1™ health watch for consumer use. The first of its kind, the Masimo W1 offers accurate, continuous measurements and insightful health data – from the leader in hospital pulse oximetry – in a personal, lifestyle-friendly, wrist-worn wearable. Building on Masimo’s decades of leadership in creating revolutionary noninvasive blood parameter monitoring solutions, Masimo W1 provides for the first time in a watch format, accurate, continuous monitoring of oxygen saturation (SpO2), as well as pulse rate, respiration rate, and more, and, in a limited market release, hydration index – for consumers wanting to better understand their overall health, improve their fitness, or share their health data with friends and family. Taking 86,400 measurements a day for second-by-second trending, the Masimo W1 watch represents the future of personal health.
Known for its exceptional accuracy and reliability during challenging conditions, such as motion and low perfusion, Masimo is bringing its expertise in signal processing, photonics, and bio-sensing to consumers looking to take control of their personal health, make better health decisions, and monitor their overall physiological status. Paired via secure Bluetooth® to the Masimo Health™ smartphone app, Masimo W1 provides continuous health data, unlocking meaningful, actionable insights, with accuracy unheard of in a wearable or watch.
Launching alongside the Masimo W1 is Personal SafetyNet™, a paid subscription service integrated within the Masimo Health app that gives users access to sophisticated reporting tools to help them review their physiological status over time. In addition, Personal SafetyNet facilitates sharing data with family members, fitness trainers, wellness coaches, and where allowed, healthcare providers, and even allows users the ability to set up virtual visits with doctors.
The Masimo W1 for consumers will be available for direct-to-consumer purchase starting immediately at www.masimo.com/masimo-w1. Masimo W1 comes with the Personal SafetyNet subscription service for consumers. Besides arterial oxygen saturation and pulse rate, Masimo W1 can also measure respiration rate, pleth variability index (PVi®), perfusion index, pulse rate variability (PRV), heart rate variability (HRV), steps, and, under limited market release terms, Hydration Index (Hi™). As part of a future update, Masimo W1 will also be able to measure temperature and VO2Max and provide 24/7 health data tracking and oversight.
Hydration level has been one of the most sought out parameters by athletes, vocalists, and others seeking to optimize their performance. Since creating PVi – which allows clinicians to assess fluid responsiveness of mechanically ventilated patients – nearly 15 years ago, Masimo has been working to invent a way to bring that measurement to consumers and those not on ventilators. Proper hydration is widely recognized as an important aspect of health and performance, and lack of proper hydration affects many physiological parameters, as the body works to restore homeostasis. Masimo W1 establishes your hydration baseline, helping you understand your hydration level, which not only affects athletic performance and fatigue, but can carry significant risks, especially for users with conditions like congestive heart failure. Whether you're an elite athlete, a vocalist, living with a chronic illness, or just keen to gain more insight into your body's physiological status, Masimo W1 with Hydration Index represents a breakthrough solution to better understanding and management of hydration.
Olympic silver medalist Morgan Pearson commented, "Masimo W1 is a gamechanger. As a professional triathlete, I’m always looking to optimize my performance in every way possible. With Masimo W1 I can continuously monitor lots of vital signs, even my level of hydration. This essential continuous data will help me track and improve my physiological performance in the most demanding of race conditions."
Dotsie Bausch, also an Olympic silver medalist, added, "Masimo W1 delivers the next generation of accuracy in wearables. I trusted Masimo technology to catapult me onto the Olympic podium as the oldest competitor in history in my discipline. Masimo delivers superior accuracy through movement, which is the golden edge any athlete is looking for to produce their very best, every single time."
Masimo is also entering the general launch phase of a medical version of Masimo W1 for use in medical applications outside the U.S., with additional measurement capabilities such as spot-check electrocardiogram (ECG), atrial fibrillation (A-fib) detection, and more. Benefiting from Masimo's expertise in hospital connectivity and hospital automation, the medical Masimo W1 will also be available for use in telehealth and telemonitoring applications via Masimo SafetyNet® and Personal SafetyNet for healthcare providers and payers, as well as individual use. For patients taking opioids to reduce pain or recovering at home after surgery or illness, as well as patients with chronic conditions (such as heart failure, COPD, or cancer), Masimo W1 will represent a convenient, reliable remote monitoring and telehealth solution enabling hospitals and clinicians to proactively keep track of their patients' physiological status from afar, even as patients go about everyday tasks at home. A natural complement to the Masimo SafetyNet remote patient monitoring platform, Masimo W1 enables wireless transmission of patient data to the Masimo SafetyNet app and Masimo’s secure data cloud, where it will be reviewed in near-real time by remote monitoring teams in centralized locations for signs of physiological decline or sudden changes, such as falls or spikes in heart rate.
In Saudi Arabia, where Masimo W1 is already approved for use in medical applications, Dr. Bakhsh, Head of the Heart Function Unit at Prince Sultan Cardiac Center, said, "We have begun using Masimo W1 with Masimo SafetyNet for remote patient monitoring of our chronic heart failure patients. The watch is very comfortable to wear, and the continuous Masimo measurements give us confidence to help keep our patients safe."
Dr. Chaudhry, Chief Clinical Information Officer (CCIO) and Chief Information Officer (CIO) at Cambridge University Hospitals NHS Foundation Trust (CUH), said, "We have deployed Masimo Patient SafetyNet™ on our Intermediate Dependency Care Unit for continuous monitoring with pulse oximetry, and believe it has made a genuinely positive impact on the safety of our patients. My initial experience with Masimo W1, the Masimo Health app and Personal SafetyNet monitoring service has been very positive. As we go forward caring for patients, whether in the hospital or at home as part of the virtual wards we are setting up, I am sure that this technology will be an excellent addition, supporting us in the delivery of ever increasing high-quality remote care for patients underpinned by personal, timely, and accurate clinical information."
Dr. Amin, Professor of Medicine and Endowed Chair of Medicine at the University of California, Irvine, noted, "Masimo continues to innovate elegant noninvasive solutions to complex problems. The release of the Masimo W1 watch raises the bar on home wearables by providing pulse oximetry based on industry-leading SET® technology and continuous monitoring features in a wrist-worn device. By doing so, we can follow our health and fitness beyond the home, to any location that has cell phone connectivity. I have personally worn Masimo W1 and am impressed by its comfort and stylish look – while able to continuously monitor my oxygen level, follow my step count, and track other health parameters, both at rest and during activity."
Joe Kiani, Founder and CEO of Masimo, said, "With over 30 years of experience in medical monitoring and telemedicine, we are excited to bring the first wearable device to offer accurate and continuous pulse oximetry, hydration index, and other health measurements to consumers. The SET® technology we invented for hospitals transformed patient monitoring, saved lives, and reduced the cost of care. Based on the feedback we have received from those who have tested Masimo W1 during the limited market release phase, we believe this watch will improve lives."
In the U.S., Masimo W1 for use in medical applications is pending FDA clearance.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2022-23 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://professional.masimo.com. Published clinical studies on Masimo products can be found at https://professional.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo W1™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo W1, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Randomized Controlled Study Finds Masimo SedLine® Brain Function Monitoring Can Help Guide Anesthesia in Children Undergoing Minor Surgery
Anesthesiologists Used Less Sevoflurane to Maintain Appropriate Anesthesia in Children Monitored with Masimo SedLine
Neuchatel, Switzerland - July 20, 2022 - Masimo (NASDAQ: MASI) today announced the findings of a randomized, controlled trial published in the Journal of Clinical Anesthesia in which Dr. Melody H.Y. Long and colleagues from the KK Women's and Children's Hospital in Singapore evaluated the ability of electroencephalogram (EEG)-guided anesthesia, using Masimo SedLine® brain function monitoring, to reduce the amount of the drug sevoflurane needed to maintain anesthesia in pediatric patients undergoing minor surgery.1 They found that use of SedLine to guide anesthesia reduced sevoflurane requirements and led to a reduced incidence of burst suppression, which has previously been reported to be associated with postoperative delirium.2-7
Noting the unique nature of pediatric brains, which are still developing, the importance that standard anesthesia practice places on minimizing the dosage of drugs needed to maintain anesthesia, and the lack of research into the use of new technology like real-time EEG spectrogram monitoring in children, the researchers devised a study that would investigate what impact such technology might have. They enrolled 195 children, aged 1 to 6 years, who were scheduled for minor surgery involving general anesthesia induced and maintained using sevoflurane. The children were randomized into either a Masimo SedLine EEG-guided group (n=100) or a standard care group (n=95). In the SedLine EEG group, anesthesiologists used SedLine to help guide administration of sevoflurane, with the goals of maintaining continuous slow/delta oscillations on the raw EEG and spectrogram, avoiding burst suppression, and maintaining a Patient State Index, or PSi – a propriety, processed EEG parameter developed by Masimo – between 25 and 50. In the standard care group, clinicians were blinded to the EEG data.
As their primary outcome, the researchers looked at the average end-tidal concentration of sevoflurane used during induction and maintenance of anesthesia. They found that in the EEG group, the concentration was lower both during induction (4.80% compared to 5.67% in the control group, p=0.003) and maintenance (2.23% vs. 2.38%, p=0.005). As one of their secondary outcomes, the researchers compared the incidence and duration of intraoperative burst suppression, and found that the EEG group had a lower incidence of burst suppression (3.1% vs. 10.9% in the control group, p=0.0440).
The authors concluded, "This is one of the first randomized control trials in the pediatric population showing that EEG-guided anesthesia care utilizing the spectrogram is feasible, and leads to a modest decrease in intraoperative sevoflurane dosage for induction and maintenance in young children aged 1 to 6 years. EEG guidance allows easy visualization of anesthesia-induced changes on the brain in real time, making it possible to determine which individuals require more (or less) anesthetic to maintain unconsciousness and titrate doses accordingly. This may be particularly important in children between 1 and 2 years old, who appear to require a higher concentration of sevoflurane during surgery, as well as in patients at risk of neurological injury. Our findings highlight the importance of EEG monitoring in complementing the current ASA standard monitors, to provide personalized anesthesia care."
William C. Wilson, MD, MA, CMO and SVP of Clinical Research and Medical Affairs at Masimo, commented, "We believe the significant reduction in burst suppression noted in the EEG group – less than one-third the amount in the control group – is an important finding. In future studies with larger sample pools, this could demonstrate more profound outcome benefits."
In the U.S., SedLine is currently indicated for pediatric use without the PSi parameter.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.8 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,9 improve CCHD screening in newborns,10 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.11-14 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,15 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.16 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Long MHY, Lim EHL, Balanza GA, Allen JC, Purdon PL, and Bong CL. Sevoflurane requirements during electroencephalogram (EEG)-guided vs. standard anesthesia care in children: A randomized controlled trial. J Clin Anesth. 27 Jun 2022. DOI: https://doi.org/10.1016/j.jclinane.2022.110913.
- Tang CJ, Jin Z, Sands LP, Pleasants D, Tabatabai S, Hong Y, Leung JM. ADAPT-2: A Randomized Clinical Trial to Reduce Intraoperative EEG Suppression in Older Surgical Patients Undergoing Major Noncardiac Surgery. Anesth Analg 2020; 131(4):1228-1236.
- Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesthesia. (2013) 110:98–105. doi: 10.1093/bja/aet055.
- MacKenzie KK, Britt-Spells AM, Sands LP, Leung JM. Processed electroencephalogram monitoring and postoperative delirium: a systematic review and meta-analysis. Anesthesiology. (2018)129:417–27. doi: 10.1097/ALN.0000000000002323.
- Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. (2010) 85:18–26. doi: 10.4065/mcp.2009.0469.
- Whitlock EL, Torres BA, Lin N, Helsten DL, Nadelson MR, Mashour GA, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. (2014) 118:809–17. doi: 10.1213/ANE.000000000000002.
- Xu N, Li L, Wang T, Jiao L, Hua Y, Yao D, Wu J, Ma Y, Tian T, Sun X. Processed Multiparameter Electroencephalogram-Guided General Anesthesia Management Can Reduce Postoperative Delirium Following Carotid Endarterectomy: A Randomized Control Trial. Front Neurol. 12 July 2021. 12:666814. doi: 10.3389/fneur.2021.666814.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Two-part Study Finds That Remote Patient Monitoring with Masimo SafetyNet® Reduced Length of Hospital Stay for COVID-19 Patients
New Study Evaluates Masimo SafetyNet in Helping Patients Recover at Home
Irvine, California – July 5, 2022 - Masimo (NASDAQ: MASI) today announced the findings of a two-part retrospective study published in Telemedicine and e-Health in which Dr. Hemali Patel and colleagues at the University of Colorado and UC Health (UCH) in Aurora, Colorado evaluated the impact of remote patient monitoring (RPM) of COVID-19 patients using Masimo SafetyNet® on hospital length of stay (LOS). Masimo SafetyNet, a remote patient management and telehealth solution, uses tetherless Masimo Radius PPG™ SET® pulse oximetry and a smartphone app to seamlessly transmit continuous home-based patient monitoring data to hospital clinicians. The researchers found a significant association between briefer hospitalization and patients discharged with Masimo SafetyNet and without home oxygen, concluding that "Home telemonitoring after discharge for patients with COVID-19 may be a safe tool that may reduce the mean duration of hospitalization and create more bed capacity."1
Noting the rising demand for acute care and hospital bed space during the COVID-19 pandemic, the researchers sought to evaluate whether use of a home telemonitoring system could help to optimize care – including earlier discharge from the hospital – for patients with or suspected to have COVID-19 while ensuring the "sustainability of health care capacity and resources" for those with more urgent needs, as well as decreasing usage of personal protective material, reducing pressure on personnel, and minimizing the risk of viral transmission. From March to June 2020, during the first COVID-19 surge at UCH, they implemented an RPM feasibility program with Masimo SafetyNet (study phase 1), which they redeployed during their second surge, October 2020 to February 2021 (study phase 2). Using Masimo SafetyNet with Radius PPG allowed them to remotely monitor oxygen saturation (SpO2), pulse rate, and plethysmographic respiration rate (Masimo RRp®) from a continuously staffed virtual health center, with data transmitted via Masimo's HIPAA-compliant secure cloud service.
Retrospectively, the researchers reviewed data for all patients discharged home after an admission for COVID-19 over the two periods. Patients discharged with and without Masimo SafetyNet were compared using a two-to-one-matched case-control design, with patients matched in each time period based on age, sex, Charlson comorbidity index, and limited English proficiency. The primary outcome was hospital LOS, and secondary outcomes were a) 7-, 14-, and 30-day hospital readmission and b) return to the emergency department within 30 days. Hypothesizing that the association between LOS and use of Masimo SafetyNet might vary according to whether patients were discharged with home oxygen therapy, they also included an interaction term for the therapy in their statistical analysis. In total, they enrolled 923 patients in phase 1 (78 discharged with Masimo SafetyNet, 845 without) and 1056 patients in phase 2 (125 discharged with Masimo SafetyNet, 931 without).
The researchers found that there was a decrease in LOS for patients discharged with Masimo SafetyNet, without an increase in 30-day ED revisits or hospital readmission (from 9.1 ± 12.4 days to 7.5 ± 5.5 days in phase 1, p=0.2721; from 6.4 ± 6.4 days to 6.1 ± 5.6 days in phase 2, p=0.6913). While there was not a significant association compared to patients discharged without Masimo SafetyNet, they describe the decrease as a "trend toward an overall lower LOS." However, as hypothesized, they observed an interaction effect between Masimo SafetyNet and home oxygen therapy: in phase 2, a decrease in LOS was strongly associated with Masimo SafetyNet patients discharged without home oxygen therapy, compared to non-Masimo SafetyNet patients – LOS decreased by an additional 36%. No other significant interactions were detected.
In their discussion, the researchers note that their study supports other work suggesting that home telemonitoring may allow for earlier hospital discharge, especially as "any reduction in length of hospitalization makes more beds available for patients." They did not find a strong association between reduction in LOS and patients discharged with Masimo SafetyNet and with oxygen therapy, which they believe makes sense "because hypoxia portends a poorer prognosis and often a longer hospitalization."
The authors concluded, "Our results remain relevant as we face yet more surges of admissions of patients with COVID-19. Even if the surges decrease, we face hospital capacity difficulties as we navigate ongoing COVID-19 admissions in addition to providing care for non-COVID patients. Home telemonitoring after discharge for patients with COVID-19 may be a safe tool that may reduce the mean duration of hospitalization and create more bed capacity."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Patel H, Hassell, A, Keniston A, Davis C. Impact of Remote Patient Monitoring on Length of Stay for Patients with COVID-19. Telemedicine and E-Health. 2022. DOI: 10.1089/tmj.2021.0510.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet®, Radius PPG™, and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, Radius PPG, and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Finds That Masimo PVi® May Be Useful in Helping ER Doctors Determine the Severity of Asthma Attacks in Pediatric Patients
Researchers Said Masimo PVi Can Be Used As a “Noninvasive, Rapid, and Objective Tool” for Helping Clinicians Predict Response to Treatment and Follow Up in Children with Obstructive Respiratory Disease
Neuchatel, Switzerland – May 23, 2022 - Masimo (NASDAQ: MASI) today announced the findings of a retrospective study published in the American Journal of Emergency Medicine in which Dr. Gülsah Demir and colleagues at Tepecik Research and Training Hospital in Izmir, Turkey investigated the potential ability of Masimo PVi® to guide emergency room triage decisions for pediatric patients with signs of obstructive respiratory disease, such as an asthma attack. PVi, or pleth variability index, is a noninvasive measurement of changes in perfusion index that occur during one or more respiratory cycles. The researchers concluded that “Automatic PVi measurement can be used as a noninvasive, rapid, and objective tool in the emergency department triage of patients admitted to the pediatric emergency department with signs of asthma attack or reactive respiratory tract disease.”1
Acute asthma attack is a common cause of admission to emergency departments (EDs) among children, and triage by severity is important for determining appropriate clinical treatment.2,3 Noting that PVi has been shown to be an accurate method for measuring the degree of pulsus paradoxus,4,5 a reduction in systolic blood pressure associated with obstructive respiratory tract disease, the authors sought to understand whether PVi might be of assistance to ED clinicians needing to make rapid triage decisions in such cases. They enrolled 133 patients between the ages of 2 and 18 (median age 5 years old) who arrived at the ED between May 2020 and July 2021 with a diagnosis of asthma attack or reactive respiratory tract disease. During initial examination and after treatment, patients’ PVi levels were measured using Masimo Radical-7® Pulse CO-Oximeters®. Severity of the asthma attack/respiratory disease was graded using the Pulmonary Index Score (PIS). Treatment decisions, including to hospitalize or to discharge, were made by clinicians who were blinded to PVi values.
The researchers found that PVi values, both before and after treatment, were significantly higher in patients with “severe” disease, compared to “mild” or “moderate” disease (p < 0.001): “Severe” patients had median PVi values of 47% (42 – 51) before treatment and 38% (32 – 44) after treatment; “moderate” patients, 31.5% (26 – 39) before and 25% (20 – 29) after; and “mild” patients, 24% (19 – 27) before and 19.5% (17 – 22) after. PVi values were also significantly higher for patients who were hospitalized, compared to those who were discharged from the ED (p < 0.001): Hospitalized patients had median PVi values of 46.5% (39 – 49) before treatment and 38% (29 -44) after treatment; discharged patients, 26% (22 – 34) before and 21% (18 – 26) after. For all severity levels, post-treatment PVi values were significantly lower than pre-treatment values (p < 0.001). The researchers calculated a pre-treatment PVi cut-off level of 37.5% for predicting whether a patient had “severe” disease (68.75% positive predictive power, with 100% sensitivity and 85% specificity, p < 0.001) and would require hospitalization (72.34% positive predictive power, with 91.89% sensitivity and 71.74% specificity, p < 0.001).
The authors concluded, “The severity of attacks of many patients who apply to the pediatric emergency department because of an attack due to obstructive respiratory tract diseases can be accurately defined with certain clinical evaluation tools. Although PIS is a very important tool for assessing attack severity, it includes some subjective parameters. Automatic PVI measurement can be useful in intensive emergency department conditions, especially in triage, in terms of predicting the response of patients to treatment and follow-up results by quickly determining the severity of attacks, and in terms of reducing subjective clinical decision variations among physicians. This is because it provides objective data.”
In the U.S., PVi is cleared as a noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.6 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,7 improve CCHD screening in newborns,8 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.9-12 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,13 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.14 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies, whose brand include Bowers & Wilkins®, Denon®, Polk Audio®, Marantz®, Definitive Technology®, Classé®, and Boston Acoustics®. Additional information about Masimo and its products may be found a https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Demir G, Berksoy E, Bardak S, Elibol P, Çiçek A, Özon A, Nalbant T, Gölkap G. Use of the pleth variability index in children with obstructive respiratory disease. Amer J Emerg Med. 11 Mar 2022. DOI: https://doi.org/10.1016/j.ajem.2022.03.019.
- Network, B.T.S.S.I.G. British guideline on the management of asthma [Internet]. 2016 [cited 2019 June26]. Available from: https://www.brit-thoracic.org.uk/qualityimprovement/guidelines/asthma/
- Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622‐39.doi: 10.1183/13993003.00853-2015.
- G Krishnan S, Wong HC, Ganapathy S, Ong GY. Oximetry-detected pulsus paradoxus predicts for severity in paediatric asthma. Arch Dis Child. 2020 Jun;105(6):533-38. doi: 10.1136/archdischild-2019-318043.
- Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department. Pediatr Emerg Care. 2018;34(10):702-5. doi: 10.1097/PEC.0000000000000887.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique technologies, including PVi, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo O3® Receives FDA Clearance for Absolute Regional Oxygen Saturation for Somatic Sites
Absolute Somatic Accuracy Enables Clinicians to Monitor Organ Tissue Oxygen Saturation with Greater Confidence Than Ever
Irvine, California – May 10, 2022 – Masimo (NASDAQ: MASI) today announced the FDA 510(k) clearance of the absolute accuracy of O3® regional oximetry for use in monitoring adult somatic tissue oxygen saturation. With this clearance, O3 is now indicated for use in monitoring both absolute and trending regional oxygen saturation (rSO2) in the U.S. in both cerebral and somatic applications for adults. Unlike somatic oximetry solutions that are indicated only for trending measurement, O3 with absolute rSO2 offers clinicians greater confidence that displayed values correlate with tissue oxygenation.
O3 regional oximetry continuously monitors the oxygen saturation of hemoglobin in the tissue under the sensor – whether in the brain or another tissue – with absolute accuracy specifications of 5% ARMS* (adult somatic, pediatric cerebral) and 4% ARMS (adult cerebral) and a trending specification of 3% ARMS (cerebral and somatic, all ages) through the use of O3 multi-wavelength sensors and O3 near-infrared spectroscopy (NIRS) technology. Unlike peripheral pulse oximetry, which reflects the body's general arterial blood oxygenation, O3 provides information about the hemoglobin oxygen saturation in local tissue, whether cerebral or somatic tissue. The absolute accuracy specifications were validated by comparing O3 rSO2 values to arterial and venous saturation values obtained via blood draw. With the clearance for absolute accuracy of somatic rSO2, clinicians can now monitor not only the direction of changes in a specific tissue's level of oxygen saturation (whether rSO2 is rising or falling) but also an rSO2 value established through comparison to a blood reference – information that may provide additional insight into a patient’s local oxygenation during various surgeries.
Monitoring both brain and somatic tissue oxygenation simultaneously has been found to further improve clinicians' ability to provide rapid and accurate care.1 André Denault, MD, PhD, Department of Anesthesiology, Critical Care Program at the Montreal Heart Institute and Central Hospital of the University of Montreal, said, "There is a growing interest in the use of somatic NIRS owing to the association of cerebral and somatic desaturation with unfavorable outcomes in shock states. As an addition to O3 cerebral oximetry, the somatic component could serve as an earlier warning of impaired tissue perfusion. Somatic NIRS has been validated as a monitor of peripheral perfusion and shows an excellent correlation with peripheral perfusion compared with radionuclide plethysmography. As previously reported,2 cerebral and somatic NIRS combined with bedside whole-body ultrasound can help in early detection of different types of shock to formulate proper therapeutic strategies."
O3 seamlessly integrates with the Root® Patient Monitoring and Connectivity Platform, a powerful, expandable monitor that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root's plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3, SedLine, and other measurements, such as SET® Measure-through Motion and Low Perfusion™ pulse oximetry, providing clinicians with expanded visibility of oxygenation status. Additional modalities available on Root include advanced rainbow® noninvasive measurements such as total hemoglobin (SpHb®) and PVi® (an indicator of fluid responsiveness), NomoLine® capnography, and more – all via an easy-to-interpret, customizable display. Using Root in combination with the Masimo Hospital Automation™ platform, monitoring data from O3 can be automatically charted in electronic medical records (EMRs). The O3 module is also available through the integration into OEM partner monitors.
Joe Kiani, Founder and CEO of Masimo, said, "Keeping an eye on the precise oxygenation status of individual organs can be critical in the operating room. This additional indication for O3 further expands its utility, giving clinicians more confidence that displayed somatic rSO2 values have been established in comparison to a blood reference. Now more than ever, we believe that O3 represents an important resource for helping clinicians and researchers better understand how the body utilizes oxygen."
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Lecluyse et al Desaturation in the Intensive Care Unit: Preliminary Experience and Review. J Cardiothoracic Vascular Anesth. (2017) 1805-1809.
- Denault A et al. A Practical Approach to Cerebro-Somatic Near-Infrared Spectroscopy and Whole-Body Ultrasound. J Cardio Vasc Anesth. 2019;03.039. DOI: https://doi.org/10.1053/j.jvca.2019.03.039
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including O3, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Medical Monitoring Pioneer Announces the Limited Market Release of the Masimo W1™ Watch for Consumers
On Its Anniversary, The Inventor of Measure-through Motion and Low Perfusion™ Pulse Oximetry Introduces the First Health Watch to Offer Accurate, Continuous Measurements
IRVINE, CALIFORNIA - May 2, 2022 – Masimo marks its 33rd anniversary today by announcing the limited market release of the W1™ health watch for consumers. The first of its kind, the Masimo W1 offers accurate, continuous measurements and actionable health insights – from the leader in hospital pulse oximetry – in a personal, discreet, lifestyle-friendly wrist-worn wearable. Building on Masimo's decades of leadership in creating revolutionary noninvasive blood parameter monitoring solutions, W1 provides accurate, continuous monitoring of multiple health parameters – including oxygen saturation (SpO2), pulse rate, perfusion index, PVi®, and respiration rate, alongside step count and fall detection.
Incorporated on May 2, 1989 as a garage startup dedicated to solving the "unsolvable" problem of inaccurate and unreliable conventional pulse oximetry under real-life conditions such as patient movement, Masimo and its breakthrough Measure-through Motion and Low Perfusion™ SET® pulse oximetry today touch hundreds of millions of lives around the world each year.1 SET® pulse oximetry has been shown in more than 100 independent studies to outperform other pulse oximetry technologies,2 and is the only pulse oximetry shown in numerous large studies – involving more than 300,000 infants – to improve critical congenital heart disease (CCHD) screening in newborns.3-13 SET® pulse oximetry has also been shown to improve outcomes for patients on opioids in post-surgical wards,14-17 reduce eye damage and blindness in the neonatal intensive care unit,18 and reduce mortality among COVID patients remotely monitored at home.19 Through Masimo's continued focus on innovation and improvement, SET® has evolved to feature the industry's highest accuracy specifications, on the new RD line of patient sensors; become tetherless, with the secure Bluetooth®-equipped continuous Radius PPG™; and now, as the foundational technology driving the W1, has become a truly lifestyle-friendly technology for consumers outside hospitals.
For the limited market release of W1, Masimo is inviting a select group of early adopters to help evaluate and refine the product over the coming months. Masimo will provide up to 10,000 W1s on a first-come, first-served basis, at a 50% discount, to users who agree to the program details and to provide feedback and data to Masimo. For additional information and to express interest in the program, please go to www.masimo.com/w1.
With this consumer release of W1, Masimo is bringing its expertise in medical monitoring, connectivity, and automation to consumers looking to take control of their personal health, including those wanting to fine-tune their athletic training and recovery, the quality of their sleep, and their overall physiological status. Paired via secure Bluetooth to the Masimo Personal Health smartphone app, W1 provides continuous health data and guidance with accuracy heretofore unknown in a wrist-based device, unlocking meaningful, actionable insights – all in the convenient and discreet form of a durable watch.
Tommy Haas, former professional tennis player, commented, "I've always believed in the power of data to improve my performance. Accurate vital sign measurements have helped me track my activity and recovery both on and off the court. Now with Masimo W1, I have a convenient way to continuously track my vitals right on my wrist."
In addition to use by consumers, W1 is also available outside the U.S. for telehealth applications, benefiting from Masimo's expertise not only in noninvasive monitoring but in hospital connectivity and automation innovations. For patients recovering at home after surgery or illness, as well as patients with chronic conditions (such as heart failure, COPD, or cancer), W1 represents a convenient, reliable remote patient monitoring and telehealth solution enabling clinicians to keep track of their patients' physiological status from afar, even as patients go about everyday tasks at home. A natural complement to the Masimo SafetyNet® remote patient monitoring platform, W1 enables wireless transmission of patient data to the Masimo SafetyNet smartphone app and Masimo’s secure data cloud.
Dr. Abeer Bakhsh, Head of the Heart Function Unit at Prince Sultan Cardiac Center in Saudi Arabia, which has been using W1 for telehealth monitoring of patients, commented, "We have begun using Masimo W1 with Masimo SafetyNet for remote patient monitoring of our chronic heart failure patients. The watch is very comfortable to wear, and the continuous Masimo measurements give us confidence to help keep our patients safe."
Joe Kiani, Founder and CEO of Masimo, said, "As we celebrate our 33rd year, and as we embark on the next chapter of our expansion through the recent acquisition of Bowers & Wilkins, Denon, Marantz, Polk Audio and their home automation technologies, it is only fitting that we are today debuting the first wearable device to offer accurate and continuous physiological measurements based on the technology we’ve honed for use in hospitals for more than three decades. From our own original breakthrough technology, SET® pulse oximetry, to our advanced hospital monitors like Root®, to our Hospital Automation™ platform and its innovative connectivity and remote monitoring systems, to our tetherless Masimo SafetyNet remote and home patient management and telehealth solutions, and now to W1, we are excited to be able to bring our technologies directly to even more people everywhere."
The Masimo W1 is not FDA cleared.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,18 improve CCHD screening in newborns,4-13 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.14-17 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,1 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.20 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Zhao et al. Lancet. 2014;384(9945):747-54.
- Gunaratne CR et al. Sri Lanka J Child Health. 2021;50(1):04-11.
- Slitine N et al. Int J Neonatal Screen. 2020;6(53).
- Ewer AK et al. Lancet. 2011;378(9793):785-94.
- de-Wahl Granelli A et al. Acta Paediatr. 2007;96(10):1455-9.
- Meberg A et al. J Pediatr. 2008;152:761-5.
- Schena F et al. J Pediatr. 2017;183:74-79.
- Hamilçıkan S, Can E. J Perinat Med. 2018;46(2):203-207.
- Jawin V et al. PLoS One. 2015;10(9):e0137580.
- Gopalakrishnan S et al. Med J Armed Forces India. 2021;77(2):214-219.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Pronovost P, Cole M, Hughes, R. Remote Patient Monitoring During COVID-19: An Unexpected Patient Safety Benefit. JAMA. Published online February 25, 2022. doi:10.1001/jama.2022.2040.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo W1™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including W1, contribute to positive clinical outcomes and patient safety; risks that Masimo W1 fails to be available as planned; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo SedLine® Brain Function Monitoring Reduced the Use of Anesthetic Agents and Opioids in a Study on Patients Undergoing Cardiac Surgery
SedLine Was Also Associated with Reduction in Bleeding During Surgery and Shorter Duration on Mechanical Ventilation
Irvine, California – April 25, 2022 – Masimo (NASDAQ: MASI) today announced the findings of a retrospective study published in the Journal of Cardiothoracic and Vascular Anesthesia in which Dr. André Denault and colleagues at the Montreal Heart Institute and Centre Hospitalier de l’Université de Montréal investigated the impact of anesthesia during cardiac surgery guided by Masimo SedLine® Brain Function Monitoring, in particular by SedLine’s processed electroencephalography (pEEG) feature, the Patient State Index (PSi). This study is the first to primarily explore the impact of pEEG-guided anesthesia on vasoactive and inotropic drugs—drugs that affect the diameter of blood vessels and that modify the force of the heart’s contractions, respectively—in the ICU. The researchers found that pEEG-guided anesthesia was associated with a reduction in the use of such drugs, as well as less use of anesthetic agents and opioids in the OR, lower central venous pressure (CVP), less fluid administration, less intraoperative bleeding, and shorter duration on mechanical ventilation.1
Noting that pEEG-guided anesthesia may improve hemodynamic stability and that high postoperative doses of vasoactive and inotropic drugs have been associated with mortality and renal dysfunction, the researchers sought to determine whether use of pEEG-guided anesthesia might improve outcomes by reducing use of such agents during cardiac surgery and at arrival in the ICU. Their primary goal was to determine whether pEEG-guided anesthesia would be associated with reduced hemodynamic instability during cardiopulmonary bypass (CPB) separation, measured by stratifying the operation into three categories: “easy” (use of only one vasoactive or one inotropic agent), “difficult” (use of at least two different classes of agents), or “complex” (requiring a return to CPB or use of mechanical circulatory support). Their secondary goal was to determine if pEEG-guided anesthesia would lead to the hypothesized reduction in vasoactive and inotropic drug administration in the ICU, measured by vasoactive and inotropic score (VIS).
The researchers compiled a retrospective cohort of 300 adult patients who underwent cardiac surgery using CPB between 2013 and 2020 at the Montreal Heart Institute. The patients were divided into two groups, depending on whether anesthesia was guided by pEEG, which became a standard of care in 2017. Patients in the pEEG group (n=150) had their brain function monitored, from the moment they entered the OR to arrival in the ICU, using Masimo SedLine.
In the pEEG group, patients received fewer vasoactive and inotropic drugs in the first hour after ICU admission, resulting in lower VIS scores (pEEG: 5 [0-10], control: 8 [2-15], p=0.003). Being in the pEEG group reduced the odds of being in a higher VIS category by 57% (OR=0.43; 95% confidence interval: 0.26-0.73; p=0.002). In addition, in the pEEG group, several additional outcomes were lower: duration of mechanical ventilation (pEEG: 3 hours [2-4 hours], control: 4 hours [3-7 hours], p<0.001), intraoperative fluid balance (pEEG: 758 mL [351-1329 mL], control: 500 mL [300-700 mL], p=0.002), and the amount of bleeding (pEEG: 400 mL [282-500 mL], control: 500 mL [300-700 mL], p=0.002).
A lower proportion of patients experienced unsuccessful (difficult or complex) CPB separation in the pEEG group than the control group (60% vs. 72%, p=0.028). However, after adjusting for other parameters using multiple logistic regression, use of pEEG-guided anesthesia was not independently associated with successful CPB separation; instead, as the researchers note, unsuccessful separation was associated with several independent known predictors of hemodynamic complications.
The researchers concluded, “pEEG-guided anesthesia is associated with a reduction in the use of inotropic or vasoactive drugs at arrival in the ICU. In addition, its implementation was associated with lower requirements of anesthetic agents and opioids in the OR, lower CVP, fluid requirements, intraoperative bleeding, and shorter duration of mechanical ventilation. However, its use did not facilitate weaning from CPB compared to a group where pEEG was unavailable. Future research is needed to confirm these results in prospective randomized clinical trials.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet®. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Jarry S, Halley I, Calderone A, Momei M, Deschamps A, Richebé P, Beaubien-Souligny W, Denault A, Couture E. Impact of Processed Electroencephalography in Cardiac Surgery: A Retrospective Analysis. J Cardio Vasc Anesth. March 2022. DOI: 10.1053/j.jvca.2022.03.030
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique technologies, including SedLine, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New JAMA Article Highlights the Outcome and Safety Benefits of Remote Patient Monitoring During the Pandemic and Beyond
Masimo SafetyNet® Reduced Mortality Among COVID-19 Patients
Irvine, California – April 12, 2022 – Masimo (NASDAQ: MASI) today announced the findings of a Viewpoint article recently published in the Journal of the American Medical Association (JAMA) which highlighted the benefits of remote home patient monitoring, reporting in part on research that used Masimo SafetyNet®, a remote patient management solution. In the article, "Remote Patient Monitoring During COVID-19: An Unexpected Patient Safety Benefit," Peter J. Pronovost, MD, PhD, and colleagues Melissa Cole, MSN, and Robert Hughes, DO, at University Hospitals Health System (UH) and Case Western Reserve University in Cleveland, Ohio conclude that through recent technological advances in remote monitoring, a patient's physiological needs can now more often be the primary factor in determining the level of monitoring they receive, rather than their physicallocation (i.e. the monitoring capabilities of the beds in a particular hospital care area).1 By not only ensuring that patients receive the appropriate level of monitoring, but enabling lower-acuity patients to be safely and reliably monitored in the comfort of their own home, Masimo SafetyNet remote patient monitoring solutions helped keep valuable hospital beds free for higher-acuity patients and improve patient safety while doing so.
To frame their argument, the authors note that the COVID-19 pandemic has "accelerated the move to monitoring and therapy based on patient risks and needs" through a "combination of medical urgency, technology advances, and payment policy." In their article, they stress the importance of continuous monitoring throughout the patient’s hospital stay, and while still ill in the home. The authors also highlight the newly recognized benefits of this shift to monitoring based on need (not location) by demonstrating how technological advances have led to impressive positive outcomes for patients monitored at home. They note that the same "[Masimo SET®] Pulse oximeters used in hospitals can now be deployed at home with patient data relayed to smartphones, secure cloud servers, and web-based dashboards where physicians and hospitals can monitor the patient’s status in near real time." This capability not only improves patient satisfaction, but leads to better patients outcomes and can "help avoid hospitalizations."
The authors note that "A recent cost-utility analysis estimated that daily assessment and 3-week follow-up of at-home pulse oximetry monitoring was projected to be potentially associated with a mortality rate of 6 per 1000 patients with COVID-19, compared with 26 per 1000 without at-home monitoring. Based on a hypothetical cohort of 3,100 patients, the study projected that remote monitoring could potentially be associated with 87% fewer hospitalizations, 77% fewer deaths, reduced per-patient costs of $11,472 over standard care, and gains of 0.013 quality-adjusted life-years."2 Masimo SafetyNet with SET® pulse oximetry and Radius PPG™ was used in the study. In another study of 33 severe COVID-19 patients discharged home, telemonitoring was found not only to be "safe, user friendly, cost-effective," but to reduce hospitalization by a mean of 6.5 days for patients requiring home oxygen.3
The researchers outline a series of steps they believe public health agencies and health systems should take to effectively encourage and implement remote patient monitoring. In conclusion, they note, "Home monitoring and hospital at-home models offer the potential to transform care and potentially allow a substantial proportion of hospitalized patients to receive care from home. Yet health systems will need to collaborate with technology companies to accelerate learning and produce greater value for patients, clinicians, and health care organizations."
Dr. Peter Pronovost, Chief Quality and Clinical Transformation Officer at UH and Clinical Professor of Anesthesiology and Perioperative Medicine at Case Western Reserve School of Medicine, said, "We could not have dreamed of remote monitoring if we didn't have the reliability of Masimo SET® pulse oximetry to provide us with accurate measurements of arterial blood oxygen saturation and pulse rate. Prior to the advent of Masimo SET® pulse oximetry, pulse oximeters were fraught with inaccurate measurements and false alarms, especially on active patients. With reliable pulse oximetry and telemonitoring, patients can now be monitored based on risks and needs rather than location in the hospital."
"Home monitoring and hospital at-home models offer the potential to transform care and potentially allow a substantial proportion of hospitalized patients to safely receive care from home," continued Dr. Pronovost.
Joe Kiani, Founder and CEO of Masimo, said, "We are proud to collaborate with health systems around the world to share the benefits of Masimo SafetyNet and our other monitoring solutions with as many patients and communities as possible. We worked with Dr. Peter Pronovost and his colleagues closely to release Masimo SafetyNet early in the pandemic, in an effort to help clinicians combat COVID-19 through remote monitoring of quarantining and recovering patients safely and reliably at home, at a time when hospitals were experiencing dramatic surges in patient volume. We have been heartened to find that the combination of clinically proven Masimo SET® pulse oximetry, tetherless Radius PPG, advanced connectivity, our secure cloud offering, and streamlined automation has helped clinicians improve outcomes and save lives."
University Hospitals and Masimo will be conducting a joint webinar to discuss the JAMA article and the benefits of remote patient monitoring on May 12 at 12:00 pm ET.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Pronovost P, Cole M, Hughes, R. Remote Patient Monitoring During COVID-19: An Unexpected Patient Safety Benefit. JAMA. Published online February 25, 2022. doi:10.1001/jama.2022.2040.
- Padula WV, Miano MA, Kelley MA, et al. A cost-utility analysis of remote pulse-oximetry monitoring of patients with COVID-2019. Value in Health. Published online October 22, 2021. doi:10.1016/j.val.2021.09.008 2014. 5:9. 2.
- Grutters LA, Majoor KI, Mattern ESK, Hardeman JA, van Swol CFP, Vorselaars ADM. Home telemonitoring makes early hospital discharge of COVID-19 patients possible. J AmMed Inform Assoc. 2020;27(11):1825-1827. doi:10.1093/jamia/ocaa168.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet® and the JAMA article based on research using Masimo SafetyNet ("the Article"). These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SafetyNet, contribute to positive clinical outcomes and patient safety; risks that the researchers' conclusions and findings may be inaccurate; risks that Masimo fails to conduct a joint webinar to discuss the Article on May 12, 2022; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Masimo
Evan Lamb
(949) 396-3376
[email protected]

Masimo Closes Acquisition of Sound United
Leading Developer of Premium Consumer Sound and Home Integration Technologies Becomes Masimo Subsidiary
Irvine, California – April 12, 2022 – Masimo (NASDAQ: MASI) today announced that it has successfully completed the previously announced acquisition of Sound United, a leading consumer technology company and owner of multiple premium audio and home entertainment brands. Sound United will operate as a division of Masimo, under its existing leadership, from its headquarters in Carlsbad, California. Sound United operates iconic consumer brands including Bowers & Wilkins®, Denon®, Polk Audio®, Marantz®, Definitive Technology®, Classé®, and Boston Acoustics®. Sold worldwide, these brands are linked by a commitment to the highest production standards and a focus on unparalleled quality and performance.
Joe Kiani, Founder and CEO of Masimo, said, “We are thrilled to add Sound United’s premium technology, established consumer channels, and well-known brands to Masimo’s broad portfolio of hospital and home medical technology solutions. We believe Masimo’s expertise in advanced signal processing, biosensing, and photonics technologies combined with Sound United’s audio and home automation technologies will bring about natural and yet non-intuitive solutions to people around the globe in home and in hospitals. Masimo will leverage Sound United’s expertise across consumer channels to accelerate distribution of the combined company’s expanding portfolio of consumer-facing healthcare products. We welcome the incredibly talented and dedicated teams at Bowers & Wilkins, Denon, Marantz, Polk Audio, HEOS, Definitive Technology, Classé, and Boston Acoustics to Masimo.”
“While we continue to identify growth opportunities by leveraging the strengths and resources from both companies, we want to express our continued commitment to our loyal customers who rely on the Sound United brands to continue driving their businesses with best-in-class solutions and forward-thinking innovation,” said Kevin Duffy, CEO of Sound United. “With our track record of industry-first innovation, superior manufacturing, and a global distribution network, we are confident that Sound United is the ideal partner for Masimo as they transform and enrich the consumer healthcare experience.”
Financial guidance associated with the Sound United acquisition will be provided during Masimo’s first quarter earnings release on Tuesday, May 3, 2022.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Sound United
Sound United, a Masimo company, was founded in 2012 with a simple mission – to bring joy to the world through sound. Today, we are one of the world’s largest portfolio audio companies and home to several legendary audio brands—Denon®, Marantz®, Bowers and Wilkins, Polk Audio, Classé, Definitive Technology, HEOS, and Boston Acoustics®. Each brand boasts its own philosophy and unique approach to bringing home entertainment to life. With centuries of collective experience, Sound United oversees the design and manufacture of a diverse array of premium audio products, including loudspeakers, sound bars, AV receivers, wireless speakers, amplifiers, turntables, and headphones. We create distinct and memorable listening experiences for a wide range of consumers in more than 130 countries. For more information on Sound United and our mission, please visit www.soundunited.com.
To learn more about Sound United and its brands, visit www.soundunited.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our acquisition of Sound United; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contacts:
Masimo
Evan Lamb
(949) 396-3376
[email protected]
Sound United
Matt Whewell
[email protected]

New Study Finds That Noninvasive and Continuous Hemoglobin Improved Transfusion Management and Outcomes in Pediatric Patients
Children Monitored with Masimo SpHb® Had Less Blood Transfusion, Less Bleeding, and Shorter ICU Stays
Neuchatel, Switzerland – March 28, 2022 – Masimo (NASDAQ: MASI) today announced the findings of a retrospective study published in the Journal of Clinical Monitoring and Computing in which Dr. Ayten Saracoglu and colleagues at Marmara University in Istanbul, Turkey evaluated the impact of noninvasive and continuous hemoglobin monitoring with Masimo SpHb® on perioperative transfusion management and postoperative patient outcomes on pediatric patients undergoing fronto-orbital advancement surgery. The researchers found that pediatric patients monitored with SpHb had lower intraoperative packed red blood cell (PRBC) transfusion, less postoperative bleeding, and shorter ICU stays.1
Noting the frequency of significant hemorrhage during craniofacial reconstruction surgery, and the importance of an adequate patient blood management (PBM) policy during such surgery, the researchers sought to determine whether PBM that included noninvasive and continuous hemoglobin monitoring might improve transfusion management and outcomes for children undergoing frontal advancement surgery. For their retrospective, case-control study, they collected data for 42 pediatric patients (average age 8.6 months ± 3.9 months) with plagiocephaly or trigonocephaly who underwent surgery between 2018 and 2021, dividing them into a group of 16 patients whose perioperative PBM included noninvasive, continuous hemoglobin monitoring (SpHb group), and 26 patients who were managed conventionally (control group). The SpHb group’s hemoglobin was intraoperatively monitored using SpHb on Masimo Radical-7® Pulse CO-Oximeters®.
The researchers found that patients in the SpHb group had significantly lower intraoperative PRBC transfusion (136.3 mL ± 40.1 mL vs. 181.5 mL ± 74.8 mL, p = 0.015), less postoperative surgical site drainage (125.3 mL ± 47.7 mL vs. 185.8 mL ± 97.6 mL, p = 0.013), and shorter postoperative ICU stay (37.1 hours ± 12.0 hours vs. 64.8 hours ± 24.9 hours, p < 0.001) than patients in the control group.
The investigators concluded, “SpHb measurement in pediatric craniofacial surgery for craniosynostosis is a safe, noninvasive tool to monitor Hb values and help transfusion decision-making, when used keeping in mind bias and inaccuracies of the device. Patients with continuous SpHb monitoring had decreased intraoperative PRBC transfusions, reduced postoperative surgical site bleeding, and shorter postoperative ICU stay.”
This study adds outcomes evidence for pediatric patients to the growing literature on the value of continuous hemoglobin monitoring with SpHb. In adults, SpHb, as part of PBM programs, has been found to improve outcomes in both high- and low-blood loss surgeries, such as reducing the percentage of patients receiving transfusions,2 reducing the units of red blood cells transfused per patient,3-5 reducing the time to transfusion,6 reducing costs,7 and even reducing mortality 30 and 90 days after surgery by 33% and 29%, respectively (when combined with a goal-directed fluid therapy algorithm using Masimo PVi®).8 This evidence of SpHb’s impact on outcomes spans the globe, now representing 7 countries on 4 different continents.1-8 Today, Masimo SpHb technology supports clinicians and patient care in more than 75 countries.
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.9 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,10 improve CCHD screening in newborns,11 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.12-15 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,16 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.17 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Saracoglu A, Abdullayez R, Sakar M, Sacak B, Incekoy F, Aykac Z. Continuous hemoglobin measurement during frontal advancement operations can improve patient outcomes. J Clin Mon Comp. 7 Mar 2022. https://doi.org/10.1007/s10877-022-00813-5.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Merolle L, Marraccini C, Di Bartolomeo E, Montella M, Pertinhez T, Baricchi R, Bonini A. Postoperative patient blood management: transfusion appropriateness in cancer patients. Blood Transfus 2020; 18: 359-65 DOI 10.2450/2020.0048-20.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including SpHb, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Debuts Telehealth for Patient SafetyNet™ at HIMSS 2022 Global Health Conference
Remote Patient Monitoring Platform Enhanced with Secure, End-to-End, Multi-Way Audio and Visual Communication
Orlando, Florida – March 14, 2022 – Today at the 2022 HIMSS Global Health Conference, Masimo (NASDAQ: MASI) announced a major expansion of Masimo Patient SafetyNet™, its premier hospital remote patient monitoring and clinician notification platform, with the addition of secure telehealth capabilities – making an already powerful solution even more versatile and comprehensive.
Available via software upgrade for existing Patient SafetyNet systems, and seamlessly integrated into view stations, telehealth for Patient SafetyNet offers secure, end-to-end, multi-way audio and visual communication between the point of care (in the room or at the bedside) to central command rooms and remote clinicians – helping to improve clinical workflows and communication efficiency across the continuum of care – without interfering with the remote monitoring of patient data. As with the recently announced expansion of the Masimo SafetyNet® platform, the system integrates TODA transcoding technology from LMLabs, which dynamically adjusts bit rates based on available bandwidth to transcode live audio and video and ensure the highest quality reproduction while requiring dramatically less bandwidth. These capabilities can be hosted within existing hospital infrastructure or even reside in the cloud, helping hospitals manage resources and comply with IT requirements while supporting fast deployment and flexible scaling.
Telehealth for Patient SafetyNet offers a host of additional advanced features designed to further improve workflow efficiency and accommodate as many communication and patient scenarios as possible. The platform supports multiple simultaneous audio and visual streams, allowing numerous clinicians and specialists to communicate and collaborate in real time, regardless of location. In addition to video discussions, users can also securely chat via text, transfer files, share their screens, and use collaborative digital whiteboards, providing seamless access to patient data, notes, and discussion in whatever medium is most relevant to each case.
With its added telehealth capabilities, Patient SafetyNet enables clinicians to remotely access continuous patient monitoring data, in near real time, while simultaneously communicating via live video stream with nurses and other clinicians – helping facilitate true 24/7 management of patient events from a central location. In addition, family members and other visitors can consult with remote clinicians virtually while staying by the patient’s bedside. Specialists and intensivists can take advantage of the streamlined multi-way communication and other advanced capabilities to offer their expertise on more patient cases, more easily than ever.
Joe Kiani, Founder and CEO of Masimo, said, “We are excited to be able to introduce such meaningful enhancements to Patient SafetyNet and showcase them at HIMSS, an organization and conference dedicated to enhancing how information is managed and conveyed in healthcare spaces. Just as telehealth for Masimo SafetyNet makes it possible for clinicians and patients to meet from afar, telehealth for Patient SafetyNet allows clinicians to collaborate and communicate in sophisticated but intuitive and efficient ways, ultimately helping to improve patient outcomes and reduce the cost of care.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica®, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Patient SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Patient SafetyNet, contribute to positive clinical outcomes and patient safety; risks that the researchers’ conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces FDA Clearance of Pediatric Indication for SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor
SedLine's Advanced Depth of Anesthesia Monitoring Now Cleared for Use on Patients as Young as 1-Year-Old in the United States
Irvine, California – February 28, 2022 - Masimo (NASDAQ: MASI)announced today the FDA clearance of SedLine® brain function monitoring for pediatric patients (1-17 years of age) and the SedLine Pediatric EEG Sensor. With this clearance, the potential benefits of SedLine have been expanded to all patients one-year-old and above in the United States. Equipped with Masimo's advanced signal processing technology, SedLine helps clinicians monitor brain activity bilaterally by processing electroencephalogram (EEG) signals from Masimo's four-lead SedLine EEG sensors.
This clearance brings Masimo's bilateral brain activity monitoring to children 1 to 17 years old, in conjunction with specially sized pediatric sensors designed for easier application on smaller pediatric foreheads. Brain activity monitoring under anesthesia on pediatric patients is different from that of adults.1-2 Maintaining an appropriate depth of anesthesia is key to preventing anesthesia-related events and enabling faster recovery.3 To aid clinicians in monitoring anesthesia depth on children, SedLine features both the display of EEG signals and the Multitaper Density Spectral Array (DSA) from both sides of the brain, to provide clinicians with a more complete picture of the brain.
Dr. Cristina Verdú of Hospital Universitario La Paz, Madrid, Spain, said, "SedLine is an easy window into a child's electroencephalogram. It helps us to personalize sedoanalgesia. Now, we can choose the appropriate dose according to its effects, not just according to weight or age. But in addition to monitoring anesthetic depth, it allows us to detect warning signs such as asymmetries or seizures; it tells us what is happening to the child's brain."
Joe Kiani, Founder and CEO of Masimo, said, "SedLine is achieving for brain function monitoring what Masimo SET® did for pulse oximetry. We believe SedLine is the best and most advanced way to monitor depth of sedation, crucial to helping ensure patients with even the most challenging and the youngest brains are appropriately anesthetized. We are proud to be able to bring its benefits to children in the United States."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Davidson et. Current Anesthesiology Reports 3. 1 (2013): 57-63.).
- Cornelissen L et al. Elife 4 (2015): e06513.
- Musialowicz et al. Current Anesthesiology Reports 4. 3 (2014): 251-260.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine® and the SedLine Pediatric EEG Sensor, as well as their FDA clearance for pediatric patients. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Adds Telehealth to Its SafetyNet® Telemonitoring System
Patient Management Platform Combines Clinically Proven Continuous Remote Monitoring, Full-Featured Video-Based Virtual Consultations, EMR Connectivity, and More
Irvine, California – February 10, 2022 - Masimo (NASDAQ: MASI) today announced a major expansion of Masimo SafetyNet® that brings robust, secure video conferencing to the remote patient management and connectivity platform to offer a comprehensive telehealth and telemonitoring solution—and for patients, a better “hospital at home” experience. Now, Masimo SafetyNet allows clinicians and hospitals to schedule and conduct multi-way audio- and video-based virtual appointments with at-home patients through the Masimo SafetyNet smartphone app—while still viewing continuous and spot-check vital signs and other physiological data. By combining the power of advanced remote patient monitoring, including Masimo’s clinically proven SET® pulse oximetry technology, with telemedicine capabilities like virtual visits and the benefits of Masimo’s Hospital Automation™ platform, such as full two-way integration with hospital electronic medical records (EMRs), Masimo SafetyNet enables use of telehealth: the ability to provide full-featured remote care with virtual face-to-face meetings while simultaneously accessing a patient’s continuous physiological data.
Masimo has integrated TODA, a robust audio and video transcoding technology from LMLabs, to expand Masimo SafetyNet beyond its original remote patient management capabilities. The integrated video solution dynamically adjusts bit rates based on the available bandwidth to transcode live, secure audio and video and ensure the highest quality reproduction with dramatically less required bandwidth. For clinicians, this means they get the best quality virtual visit combined with real-time patient data, no matter where the patient is. Not only are clinicians able to communicate with their patients, but also collaborate with them with the ability to share their screen, launch a digital whiteboard, chat via in-app secure messaging, or invite additional clinicians for more expertise.
For patients, Masimo SafetyNet is now the easiest way to connect with their doctors and care team without having to download additional apps or send in their physiological measurements separately. Their virtual visits are conducted through the same smartphone app that collects their oxygen saturation, pulse rate, respiration rate, temperature, and other data from tetherless Masimo Radius PPG™ and Radius Tº™ sensors. Through the new virtual whiteboard, providers can educate and share additional information as part of the discussion, enriching patient-clinician interactions and allowing them to be tailored to meet each patient’s health and communication needs. The integrated messaging feature offers yet another way for patients to stay in touch with their care teams, when a full video consultation is not needed.
Joe Kiani, Founder and CEO of Masimo, said, “Masimo SafetyNet has been helping clinicians save and improve patients’ lives at home and in the hospital, around the world, since the start of the pandemic. With the addition of advanced telehealth capabilities, our already powerful remote monitoring solution becomes an even more comprehensive platform.”
Designed to help providers remotely manage patient care, Masimo SafetyNet is a secure, scalable, cloud-based patient management platform that features clinical-grade spot-checking and continuous measurements, CarePrograms (customizable digital care plans), remote patient surveillance, and flexible, automated, two-way integration with hospital EMR systems – now augmented with video telemedicine. First developed for use during the COVID pandemic, for lower-acuity patients recovering or quarantining at home, Masimo SafetyNet has expanded use to post-surgical patients, patients with a variety of chronic conditions, and to patients with episodic illnesses, including fever. The platform helps seamlessly extend care from the hospital to the home (or any other location outside the hospital or doctor’s office) by collecting monitoring data from the fingertip and chest-worn sensors, relayed to the patient’s smartphone with Bluetooth®, and from there to the secure Masimo SafetyNet cloud. Using the web-based clinician portal, doctors and other clinicians can keep an eye on patients’ physiological progress from afar, intervening if a patient’s condition appears to worsen, and now with the ability to conduct comprehensive virtual visits as well.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67®, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, iSirona™, Patient SafetyNet, Replica™, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Temple University Hospital Adopts Masimo Centroid™
Premier Philadelphia Academic Medical Center Implements Advanced Masimo Wearable, Wireless Patient Position, Orientation, and Activity Sensors in its ICUs
Philadelphia, Pennsylvania - February 7, 2022 - Masimo (NASDAQ: MASI) and Temple Health today announced that Temple University Hospital (TUH), a 722-bed academic medical center located in Philadelphia, is expanding its use of Masimo technologies with Centroid™, an advanced wireless patient position, orientation, activity, and respiration rate sensor, at 100 beds across its ICU units.
Centroid helps clinicians monitor patient position to avoid preventable pressure injuries, and can alert clinicians to sudden movements such as fall-like events. In addition, Centroid detects chest movements to continuously provide respiration rate, assisting clinicians with additional data that may inform care decisions. Centroid pairs with the Root® Patient Monitoring and Connectivity Platform using Bluetooth® to track a patient's posture, orientation, and activity, providing the ability to monitor patient position and detect changes in position. The data transmitted by Centroid can be displayed in various formats on Root, giving clinicians multiple ways to assess adherence to protocols regarding tissue stress and to tailor care to the specific needs of each patient. In addition, Centroid data can be relayed via the Masimo Hospital Automation™ platform to Patient SafetyNet™, Masimo’s centralized remote patient supplemental monitoring platform, and Replica®, a mobile application that allows clinicians to view continuous data regardless of location.
At TUH, all ICU beds are being equipped with Root and with Centroid, including the Trauma ICU, Cardiothoracic ICU, Burn ICU, Neurological ICU, and Medical Respiratory ICU. Angelo Venditti, DNP, RN, Executive Vice President for Patient Care and Chief Nursing Executive at Temple Health, said, "We are pleased to expand our relationship with Masimo, which has already proven itself as a key technology partner in our efforts to improve patient outcomes. When we trialed Centroid, we found it helped our teams prioritize workflows more effectively, with increased focus on following turn protocols and decreased incidence of pressure injuries."
Hospital-acquired pressure injuries (HAPI), commonly known as bed sores, are on the rise – occurring in nearly 5% of all hospitalized patients in the US.1 Elderly and critically ill patients are often at highest risk for developing a HAPI, which can lead to further treatments and extended lengths of stay in the hospital.
To compound the patient impact associated with these pressure injuries, there is a major economic burden for the hospital as well. Pressure injuries are classified as a "Hospital Acquired Condition," or HAC, and the treatment costs associated with HACs are non-reimbursable to the hospital. One pressure injury can quickly add up to tens of thousands of dollars. Many facilities in the US spend millions each year treating these wounds.2 Pressure injuries are also a reportable quality metric to CMS.3
According to the National Pressure Injury Advisory Panel (NPIAP), scheduled turning protocols are known to prevent HAPIs, yet most hospitals in the US still use techniques such as paper wall clocks or egg timers to try and optimize this practice4 – methods that are proving to be ineffective and archaic in modern healthcare. Staffing issues and competing priorities have strained nurses across the US, and staying on top of when a patient was last turned is difficult without a more sophisticated system.
Centroid is a single-patient-use adhesive sensor indicated for the orientation monitoring of patients who may be susceptible to pressure ulcers by tracking patient movement and activity using an accelerometer and gyroscope. Centroid can identify a patient's position and orientation to the nearest degree, with alerts based on the duration in a static position to help clinicians adhere to hospital patient turn protocols. Centroid also features customizable alarm zones to help avoid patient positions that could negatively impact recovery time. Unlike simple time-based rotation protocols, Centroid provides an advanced pressure risk algorithm that takes into account the cumulative pressure exposure time, with body segment-by-segment resolution, using the integrated gyroscope, displayed on the Root screen using color-coded markers. This can help clinicians not only identify if a patient has been turned, but also identify if the new position may still be resulting in pressure risk to the same tissue – and ultimately, helping to guide clinical decisions about the most appropriate, least risky positions for each patient.
Temple Health and Masimo have been pulse oximetry technology partners since 2008. Temple implemented Masimo Patient SafetyNet in 2014, has since expanded their Patient SafetyNet system to seven units, and has also adopted Masimo SpHb® noninvasive hemoglobin monitoring, NomoLine® capnography, SedLine® brain function monitoring, O3® regional oximetry, and now the Centroid patient positioning tracker.
Joe Kiani, Founder and CEO of Masimo, said, "We are honored to continue to be able to equip Temple – one of the country's premier medical institutions – with so many of our life-saving solutions, from monitoring technologies like SET® pulse oximetry and rainbow® Pulse CO-Oximetry, to advanced monitors like Root and Hospital Automation solutions like Patient SafetyNet. With the addition of Centroid, Temple adds another key Masimo innovation to their toolkit. It's a great example of our commitment to developing new ways to provide the highest quality, most relevant data to clinicians in the most intuitive, useful formats. We look forward to helping Centroid prevent many HAPIs and improve the outcomes and lives of their patients."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,6 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-11 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,12 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.13 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION®, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3217823
- https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HAC/Hospital-Acquired-Conditions
- https://pubmed.ncbi.nlm.nih.gov/30693644/
- https://cdn.ymaws.com/npiap.com/resource/resmgr/white_papers/1i._pressure_ulcers_in_indiv.pdf
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
About Temple University Hospital
Temple University Hospital's mission is to provide access to the highest quality of health care in both the community and academic settings. The 722 bed academic medical center supports Temple University and its Health Sciences Center's academic programs by providing the clinical environment and service to support the highest quality teaching and training programs for health care students and professionals, and to support the highest quality research programs. The hospital's values are simple: Respect, Service and Quality. Temple University Hospital – Main Campus (TUH) is a nationally respected academic medical center that offers high-quality care, the latest technology and skilled staff – all on a safe, easy-to-reach campus. As the chief clinical training site for the Lewis Katz School of Medicine at Temple University, TUH is a teaching hospital where the latest procedures and techniques are pioneered and where students and other physicians come to learn. It is also a robust research center that is advancing the fight against disease, pushing the boundaries of medical science and altering the course of serious disease. All of this means that Temple physicians and staff are at the forefront of medical knowledge. TUH is many things to many kinds of patients. For some, it's the hospital they depend on for their routine medical care. For others, it's a place to receive specialized, lifesaving treatments that can’t be found at most hospitals. Temple’s nationally renowned medical staff offer dozens of powerful new options for patients who, just a few years ago, were considered untreatable. So whether a patient needs a family doctor or a highly trained specialist, chances are good they can find that physician at Temple. Although surrounded by modern equipment and state-of-the-art facilities, it's notable that Temple physicians and staff never lose sight of what is most important – patients and their families. This personalized approach to care makes the hospital a true medical home for hundreds of thousands of patients each year.
Forward-Looking Statements – Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Centroid, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Retrospective Study Finds That Masimo SET® Pulse Oximetry Has No Difference in Accuracy or Bias Between Black People and White People
Irvine, California - January 17, 2022 - Masimo (NASDAQ: MASI) today announced the findings of an abstract presented last Saturday at the Society for Technology in Anesthesia 2022 Annual Meeting, which reviewed retrospective pulse oximetry data and concluded that there was no clinically significant bias based on ethnicity for black and white volunteer subjects monitored with Masimo SET® pulse oximetry and RD SET® sensors.1
A 2020 letter to the editor2 and paper3 purported to find "racial bias" in pulse oximetry measurements, based upon a comparison of data obtained from black and white patients compiled from multiple sets of health record data, using unspecified pulse oximeters and controls. Masimo Founder and CEO Joe Kiani responded to the letter and promised additional research to test Masimo's understanding of the subject and hypothesis about the performance of Masimo technology. The first of such studies was conducted and reported by Dr. Steven J. Barker (Chief Science Officer, Masimo) and Dr. William C. Wilson (Chief Medical Officer, Masimo). Drs. Barker and Wilson performed a retrospective analysis of Masimo laboratory data obtained from black and white volunteer subjects, in an effort to identify differences in Masimo pulse oximeter accuracy and bias between ethnic groups.
The authors reviewed data collected between October 2015 and July 2021, which included 7,183 paired samples (3,201 black and 3,982 white) collected from 75 subjects (39 black and 36 white), who were screened with the same criteria to remove potential bias based on health conditions. All subjects were exposed to the same hypoxia protocol, which varied the arterial saturation of hemoglobin (SaO2) between 70% and 100%. Noninvasive oxygen saturation (SpO2) values were obtained from Masimo SET® pulse oximeters with RD SET sensors and time-matched (within 5 seconds, rather than up to 10 minutes as in the letter to the editor) with arterial blood gas (ABG) samples analyzed using an ABL-835 blood gas analyzer.
The authors analyzed the data to determine the bias (the mean difference in paired SpO2 and SaO2 samples), precision (standard deviation of the difference), and accuracy (root mean squared error, ARMS*) for both groups. They found a negative bias of 0.20% for black subjects, compared to a negative bias of 0.05% for white subjects, a difference of 0.15% (p < 0.001), which is not clinically significant and is numerically indistinguishable because the SpO2 display resolution is 1% on commercially available pulse oximeters (both from Masimo and other manufacturers). They found precision of 1.40% for black subjects and 1.35% for white subjects. Accuracy (ARMS) was 1.42% for black subjects and 1.35% for white subjects. These results are consistent with the accuracy specifications of RD SET sensors (1.5% accuracy ARMS).
By comparison to the Masimo finding of 0.15% difference in bias between black and white subjects, the 2020 letter to the editor reported a difference in bias of 8.1% in a cohort of black and white hospital patients2 – 54 times higher than the Masimo result.
Masimo is conducting additional studies and will report its findings in the future.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within
+/-ARMS of the reference measurements in a controlled study.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes, reduce the cost of care, and take noninvasive monitoring to new sites and applications. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals as ranked in the 2021-22 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris® Gateway, Patient SafetyNet, Replica®, Halo ION™, UniView®, UniView :60™, and Masimo SafetyNet®. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Barker SJ, Wilson WC. Accuracy of Masimo SET® Pulse Oximetry in Black and White Volunteer Subjects: A Retrospective Review. Annual Meeting of the Society of Technology in Anesthesiology. Presented Jan 15, 2022.
- Sjoding MW, et al: Racial Bias in Pulse Oximetry Measurement. N Engl J Med 2020; 383 (25):2477-2478.
- Valbuena VSM, et al: Racial Bias in Pulse Oximetry Measurement Among Patients About to Undergo ECMO in 2019-2020, A retrospective Cohort Study. Chest 2021 Sep 27; S0012-3692(21)04065-4.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET® and RD SET®, and Masimo's plan to conduct additional studies on the accuracy of Masimo SET® and RD SET on black people and white people ("Additional Studies".) These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET® and RD SET, contribute to positive clinical outcomes and patient safety; risks that Masimo fails to conduct Additional Studies as planned; risks that the researchers' conclusions and findings may be inaccurate; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]