Capnography and Gas Monitoring Solutions
Masimo is partnering with frontline clinicians and patient monitoring companies to provide a complete portfolio of capnography and gas monitoring solutions.
Home / Capnography and Gas Monitoring Solutions
Capnography and Gas Monitoring Solutions
End-Tidal Carbon Dioxide (EtCO2) Monitoring
End-Tidal Carbon Dioxide (EtCO2) Monitoring
End-tidal carbon dioxide (EtCO2) is the concentration of carbon dioxide (CO2) at the end of an exhaled breath. The EtCO2 reflects efficiency with which CO2 is carried from its origin in metabolizing cells through capillaries to the alveoli where it is exchanged with O2, and then exhaled via conducting airways through the trachea to the nasopharynx and oropharynx where it is released from the body. EtCO2 is most accurately measured in intubated patients, and in those with tight fitting masks (no air leak during inhalation or exhalation).1 The EtCO2 is directly related with metabolism, cardiac output, and pulmonary blood flow and inversely related to dead space (not ventilated alveoli and conducting airways). Changes in EtCO2 can signal changes in cardiovascular function as well as respiratory events.1 Capnography provides:1
- A waveform showing a graphical display of CO2 levels during breathing (capnogram)
- A numerical value for EtCO2 - the exhaled CO2 value at end exhalation (top of plateau)
- Respiratory Rate (RR) derived from counting the frequency of exhaled CO2 plateaus
- A continuous measure of airway patency and ongoing cardiovascular circulation
Capnography is an important tool used by clinicians to identify alterations in ventilation and cardiac output, as well as an acute warning of certain potentially life-threatening conditions such as apnea airway obstruction, extubation, pulmonary embolus, and cardiac arrest.1
Because monitoring EtCO2 allows for a continuous evaluation of ventilatory status (including patency of breathing circuit) and adequacy of cardiac output, it is required during anesthesia for procedures,2 and increasingly used in pre-hospital emergency medical services (EMS), emergency departments (EDs), and intensive care unit (ICUs). Capnography can also be used to non-invasively monitor ventilation in spontaneously breathing patients.3
NomoLine® Capnography Portfolio
NomoLine® Capnography Portfolio
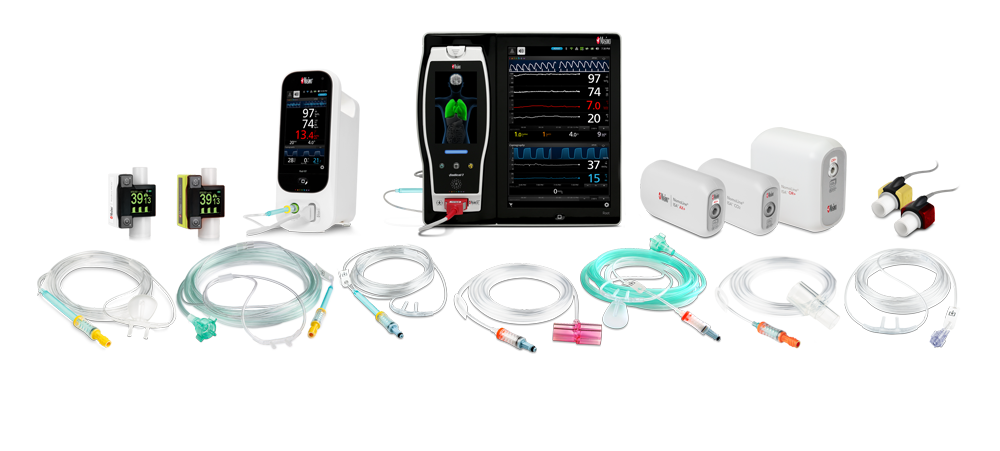
Masimo offers a complete portfolio of NomoLine capnography and gas monitoring solutions, both sidestream and mainstream, to meet the challenges of ventilation and gas monitoring across multiple clinical settings. Solutions range from external ‘plug-in and measure’ gas analyzers, bedside and handheld devices, flexible, integrated OEM offerings.
Capnography-enabled Masimo devices need virtually no warm-up time, provide full accuracy performance in ten seconds, require no calibration4, and benefit from the unique moisture-wicking technology of NomoLine sampling lines, helping clinicians streamline workflows.
NomoLine ISA™ Gas Analyzers
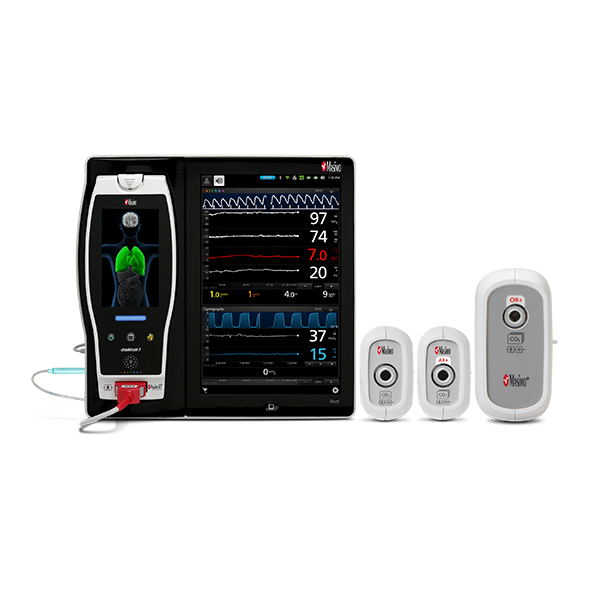
NomoLine infrared sidestream gas analyzers provide end-tidal carbon dioxide and multi-gas measurements (oxygen, nitrous oxide, and anesthetic gases) in a variety of clinical settings for all patient populations. With no calibration requirements, auto-zeroing and a simple visual indicator (LEGI), use of the ISA modules may help simplify clinician workflows. They are available as a standalone external module for use with Root®, integrated into the Rad-97® device, or as easy-to-integrate OEM modules.
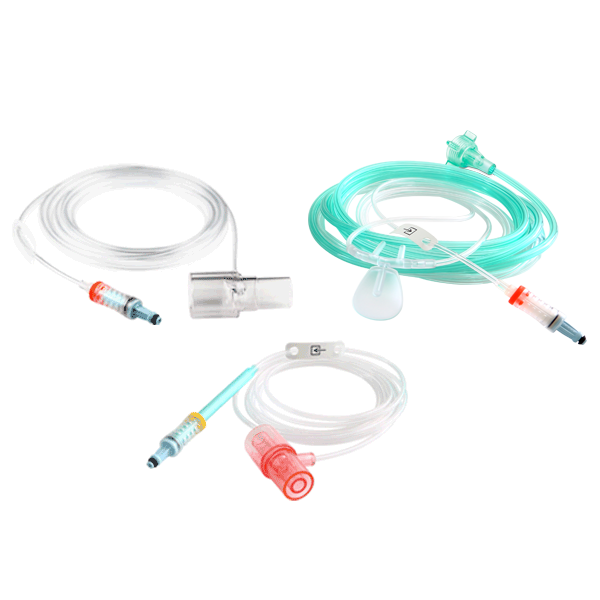
NomoLine Sampling Lines
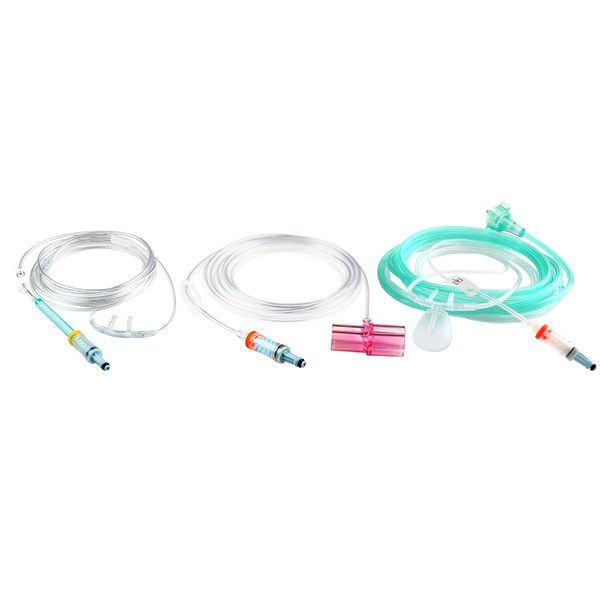
NomoLine sampling lines include airway adapter sets and cannulas for gas monitoring of all patient populations in a variety of clinical settings. They include long- and short-term sampling lines with moisture-wicking technology, providing accurate capnography and gas measurements. NomoLine sampling lines are available in a variety of configurations that are compatible with Masimo as well as third-party capnography monitors.
IRMA™ Gas Analyzers

IRMA mainstream analyzers provide waveform capnography and anesthetic gas measurements for monitoring intubated patients. Mainstream technology measures gas directly in-line and does not remove gas from the anesthesia circuit, reducing delays in measurement. Compact size and lightweight design reduce weight on the respiratory circuit, allowing for use on smaller patients. Quick start-up time of less than 15 seconds4 may allow clinicians in emergency settings to make quicker informed decisions.
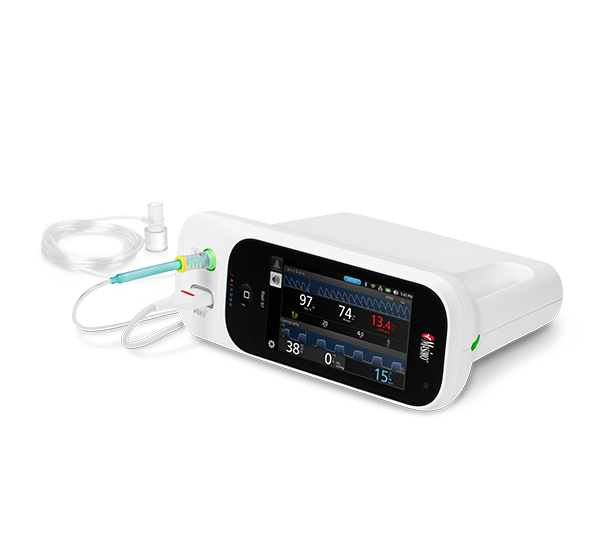
EMMA® and Radius PCG™ Capnographs

EMMA and Radius PCG are compact, portable, and lightweight mainstream capnographs that provide EtCO2 and RR measurements within 15 seconds.4 They are designed to fit easily onto a breathing circuit for short-term monitoring of intubated or ventilated patients. Radius PCG additionally provides Bluetooth® connectivity to Masimo devices such as Root, allowing for seamless transfer of patient data to the electronic medical record.
Capnography and Gas Monitoring Solutions
References:
- 1.
Lumb A: Nunn’s Applied Respiratory Physiology. 7th edition London Churchil Livingstone; 2010:174–7
- 2.
American Society of Anesthesiologists (ASA) Standards for Basic Anesthesiology Monitoring -Developed By: ASA Committee on Standards and Practice Parameters (CSPP). Last Affirmed: December 13, 2020 (last amended October 20, 2010) (original approval: October 21, 1986) - https://www.asahq.org/standards-and-guidelines/standards-for-basic-anesthetic-monitoring
- 3.
Kodali BS: Capnography Outside the Operating Rooms. Anesthesiology 2013; 118:192-201
- 4.
Operator ’s Manual
RESOURCES

Follow the link for available Capnography and Gas Monitoring Studies.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
The Bluetooth® word mark and logos are registered trademarks owned by Bluetooth® SIG, Inc. and any use of such marks by Masimo is under license.
PLCO-005898/PLM-11090C-0423