Home / SET® Neonatal Difference
SET® Neonatal Difference

No Challenge Too Big. No Patient Too Small.
Your Trusted Partner in Neonatal Care
For over 25 years, care teams have relied on Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry to deliver accurate SpO2 and pulse rate readings—even in challenging conditions where other pulse oximeters fail.
Today, we remain committed to protecting your most delicate patients and strive to deliver a variety of specially tailored solutions that meet their needs and help you improve outcomes.
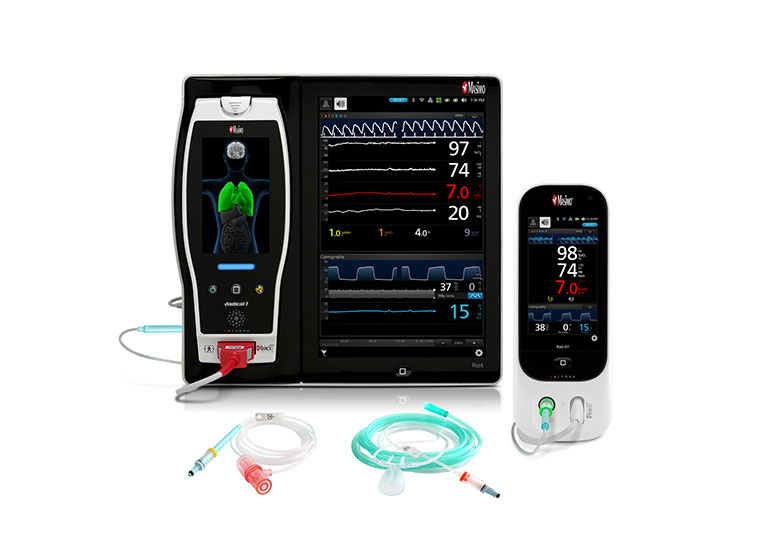
Discover the Masimo SET® Advantage
Discover the Masimo SET® Advantage
More than 10 critical congenial heart disease (CCHD) studies with SET®, representing over 300,000 babies, have shown improved screening sensitivity with the use of SET® alongside clinical assessment when compared to routine physical exam alone.1-11
In an independent, peer-reviewed study, clinicians using an oxygen targeting protocol with Masimo SET® reduced ROP rates by 10% (from 12.5% to 2.5%) over five years.12
In an independent, peer-reviewed study conducted on premature neonates with an Apgar mean score of 5, Masimo Newborn Sensors were shown to post both SpO2 and pulse rate over 45 seconds faster (mean difference) than competitor technology.13
In an independent, peer-reviewed study of neonates, Masimo SET® had 86% fewer false alarms and 92% less total alarm time compared to competitor technology.14
RD SET® sensors offer best-in-class SpO2 accuracy specifications during motion: 1.5% ARMS.*
The Masimo Blue® Sensor provides accuracy specification down to 60% SpO2 and is uniquely designed for and validated on cyanotic neonatal, infant, and pediatric patients with congenital heart disease.15
In an independent, peer-reviewed neonatal study, Masimo SET® pulse oximetry identified 86% of all bradycardic events, compared to just 14% with competitor technology.14
RD SET NeoPT-500 sensors are designed with zero adhesive components, offering a soft, lightweight, and compact solution that lies comfortably on a patient’s hand or foot.
The Project That Revolutionized Neonatal Care
The Project That Revolutionized Neonatal Care
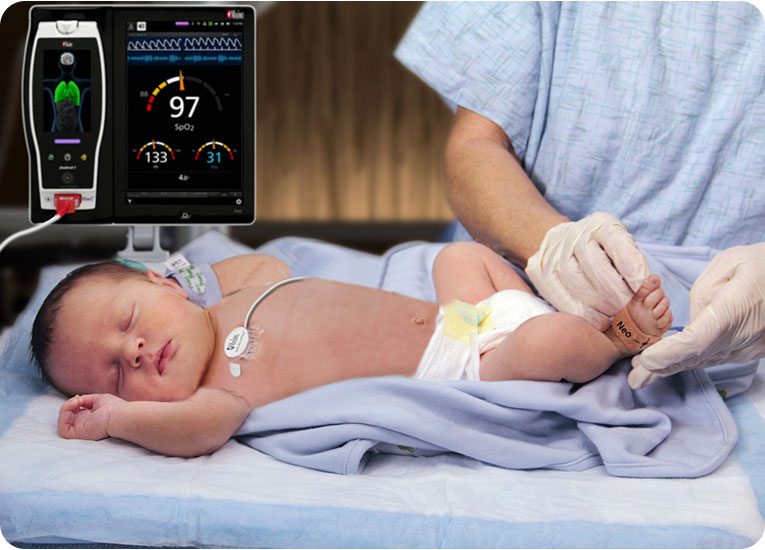
Our commitment to patient safety began with Project Stork, a 1992 proof of concept for Masimo SET® pulse oximetry that aimed to demonstrate its ability to help clinicians protect neonates at their most vulnerable: in the first moments of life. What we found was that SET®, combined with the expert knowledge and decision-making of clinicians like you, was poised to make a profound impact on neonatal care.
Watch the video above to learn more.
Discover a Range of Neonatal Monitoring Solutions
Discover a Range of Neonatal Monitoring Solutions
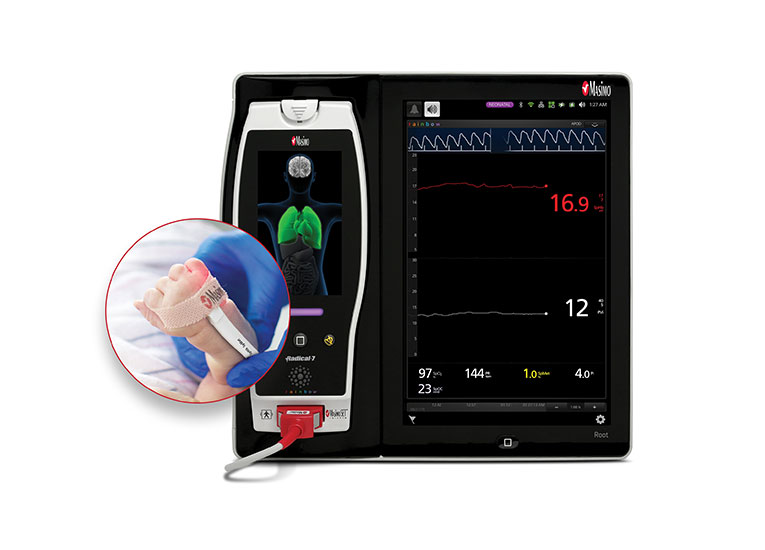
Improve Neonatal Outcomes with SET® Pulse Oximetry
Improve Neonatal Outcomes with SET® Pulse Oximetry
Fill out the form below to receive more information.
References:
- 1.
Zhao et al. Lancet. 2014 Aug 30;384(9945):747-54.
- 2.
Meberg A et al. J Pediatr. 2008;152:761-5.
- 3.
Schena F et al. J Pediatr. 2017;183:74-79.
- 4.
de-Wahl Granelli A et al. BMJ. 2009;8:338.
- 5.
Ewer AK et al. Lancet. 2011;378(9793):785-94.
- 6.
de-Wahl Granelli A et al. Acta Paediatr. 2007;96(10):1455-9.
- 7.
Slitine N et al. Int J Neonatal Screen. 2020;6(53):30.
- 8.
Gunaratne CR et al. Sri Lanka J Child Health. 2021;50(1):04-11.
- 9.
Jawin V et al. PLoS One. 2015;10(9):e0137580
- 10.
Hamilçikan S, Can E. J Perinat Med. 2018;46(2):203-207.
- 11.
Gopalakrishnan S et al. Med J Armed Forces India. 2021;77(2):214-219.
- 12.
Chow LC et al. Pediatrics. 2003;111(2):339-45.
- 13.
Baquero H et al. Acta Pediatr. 2010;100:515-518.
- 14.
Hay W et al. J Perinatol. 2002;360–366.
- 15.
Harris B. et al. Ped Crit Care Med. 2016;17(4):315-20.
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-005081/PLM-13125A-1021